Understanding, preventing corrosion in refineries
Norman P. Lieberman
Process Improvement Engineering
Metairie, La.
My major problem as an operating supervisor when I worked for the former Amoco Oil Co. refinery in Texas City, Tex., was not the people, the process, or the plant’s economics; it was corrosion. Most of the corrosion products I encountered were a black, slippery, finely divided powder. Upon drying, the black powder would autoignite, burning with a pale blue flame not visible in sunlight that emitted a white vapor, which made me choke. The white vapor, of course, was sulfur dioxide (SO2).
Analysis indicated this black powder was iron sulfide (FeHS), or pyrophoric iron. This was rather surprising, as hydrogen sulfide (H2S)—which is quite a weak acid—isn’t particularly corrosive at moderate temperatures. Why then, I wondered, was all this corrosion to piping, vessels, and heat exchangers operating at less than 200° F. due to H2S?
The source of my corrosion problem was not so much H2S as it was the real evil agent of corrosion: hydrochloric acid (HCl).
HCl in refineries
I have always considered HCl to be a rather evil molecule. In refineries, it originates in two benign ways: naturally from sea water and via manmade activities to increase octane in gasoline.
Sea water consists of 97% water and 3% salts. The salts are mainly 90% sodium chloride (NaCl), 4% calcium chloride (CaCl2), and 6% magnesium chloride (MgCl2).
The NaCl is pretty much removed in the crude unit desalter, as is much of the CaCl2. The MgCl2 is less soluble in the desalter water, and hence, 30-40% escapes from the desalter.
At 500° F. or hotter, the MgCl2 will dissociate into MgCl2 + H2O = Mg(OH)2 + HCl.
The second origin of HCl is manmade. We inject chlorides into the hydrogen recycle gas on naphtha reformers to enhance gasoline octane. The chlorides react with the recycle hydrogen to produce: H+ + CL- = HCl.
Both above reactions produce very small amounts (just a few ppm) of HCl. If it’s dry, the HCl is completely noncorrosive. Should the HCl—which is a particularly strong acid—become wet, however, it becomes terribly corrosive to carbon-steel piping, vessels, and heat exchanger tubes. The result of this corrosive reaction is: 2HCl + Fe = Fe(Cl)2 + H2.
The hydrogen is produced as a hydrogen ion. These protons (H+) dissolve into the surrounding metal walls. They get trapped at imperfections in the metallic structures, combine to form molecular hydrogen (H2), and in so doing, create localized hydrogen pressures, or stresses, inside a metal wall. This is the origin of hydrogen-assisted stress corrosion cracking, which is a leading cause of death and disaster in refineries and chemical plants.
Fe(Cl)2 is water soluble. As the HCl produced is present in only a few ppm, it does not directly result in very much metal loss except when aided by H2S in its evil effects.
HCl is a very strong acid, while H2S is a much weaker, less reactive acid. A weak acid like H2S ordinarily could never displace a strong acid like HCl from its salt (i.e., FeCl2). The concentration of H2S, however, is typically 10,000 times greater than that of HCl. Thus, the following unfortunate reaction will take place: H2S + FeCl2 = Fe(HS) + HCl.
Again, this reaction can only proceed in an aqueous phase. The resulting iron sulfide—which is insoluble in water—is that black, thin powder we find all over the planet. The HCl so liberated from the iron sulfide goes on to destroy a new molecule of iron.
In addition to being corrosive itself, hydrochloric acid is a catalyst for corrosion when mixed with water and high concentrations of hydrogen sulfide.
Preventing corrosion
In refineries, most of the origin of the corrosion I have just described can be mitigated by improved crude desalter operation to enhance Mg(Cl)2 extraction. In order of importance, here are steps to minimize MgCl2 escape from the desalter:
• Conduct double desalting.
• Inject all washwater upstream of the first crude preheat exchanger, not at the inlet of the mix valve.
• Use 6 wt % or more water on crude (4 wt % is too little).
• For heavier crudes, use desalter temperatures of 280-300° F. For lighter crudes, use desalter temperatures of 250-270° F.
• If the sour-water stripper bottoms are used as desalter washwater, keep NH3 concentration at less than 20 ppm.
• Control desalter pH to 6.5-7.5 and do not add caustic to the sour-water stripper.
• Continuous use of water recirculation through bottom wash nozzles to reduce solids buildup is helpful but not critical.
• Add 50% of chemical demulsifiers to the crude charge tank and the remainder to the desalter itself.
• Use a conductivity probe to monitor the crude-to-emulsion interface levels.
• Basic sediment and water (BS&W) should be no more than 0.2-0.3 wt %.
Reformer hydrogen
Chlorides escape from the naphtha reformer with the surplus hydrogen used in the downstream hydrotreating units. In the old days (i.e., in my time), we used a 10% caustic wash to extract HCl from the reformer offgas. Current practice is to remove HCl from the hydrogen offgas with a molecular-sieve regenerable absorption tower.
Regardless of the method used, my clients only too often neglect monitoring of the chloride-removal step from their reformer hydrogen offgas, with sometimes catastrophic downstream results due to the H2S + FeCl2 = Fe(HS) + HCl reaction. An occasional check with a Drager tube of the hydrogen offgas for chloride content by the unit engineer may prove to be an effective way to minimize death and disaster in downstream hydroprocessing units.
Carbonic acid
An excellent way to remove rusty stains from your toilet bowl is to pour a can of Coca-Cola directly over the rust. I prefer to use Diet Coke, however, as we don’t want to waste Classic Coke on this activity.
It’s the dissolved carbon dioxide (CO2) in the aqueous phase (H2CO3), or carbonic acid, that attacks the iron deposits in your toilet. Carbonic acid, unlike HCl and sulfuric acid (H2SO4), is quite corrosive to carbon steel, even at a relatively high pH. If hydrochloric acid has a pH of 5.5-6.0, that’s not too alarming. Water with the same pH due to carbonic acid, however, will be quite corrosive.
I normally encounter corrosive CO2 in an aqueous phase in steam condensate. The CO2 originates in steam due to calcium carbonates (Ca2CO3) in the steam generator boiler-feed water supply. Upon heating in the boiler, the carbonates dissociate into: Ca2CO3 + H2O = Ca(OH)2 + CO2 .
The CO2 is a noncondensable component left behind in heat exchangers after the steam condenses. As a noncondensable, it accumulates inside of the steam reboilers and the steam shell-in-tube heat exchangers. If not vented off, or neutralized, it will form high concentrations of carbonic acid in the exchanger and become very corrosive to carbon steel.
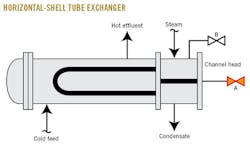
If the steam is on the tube side of a horizontal shell and tube exchanger, then the accumulated CO2 must be vented off beneath the channel-head pass partition baffle (Fig. 1, Point A). Venting from the high-point connection (Fig. 1, Point B) will only vent steam and not any of the accumulated CO2. My practice is to vent continuously from Point A through a restriction orifice sized at 0.5% of the total supply of the steam flow. I have borrowed this design feature from a representative of Shell Global Solutions International BV who attended one of my troubleshooting seminars in 1984.
At Texas City in the 1970s, we handled this accumulation of corrosive carbonic acid in the channel head of our steam-heated reboilers by injecting neutralizing amines into the reboilers’ steam supply. But we had a good reason for doing this at the Texas City refinery, as the plant manager was treated to duck hunting trips by the chemical vendors.
Other corrosive agents
Naphthenic acids and sulfur compounds may also prove to be active corrosive agents in refineries. High-corrosion rates for these elements, however, often require higher temperatures or high velocities. Most of the corrosion problems I have encountered as an operating superintendent for alkylation and crude units, as well as delayed coking plants, have been associated with aqueous phases. In general, water is the most corrosive medium I have contended with in many refinery applications, especially when combined with NH4Cl salt deposits and hydrogen sulfide vapors.
The author
Norman P. Lieberman ([email protected]) is a chemical engineer with more than 50-years’ experience in process plant operation, design, and field troubleshooting. He previously served as operations supervisor, technical service manager, and plant manager at US refineries until 1985. An independent consultant, he also has taught 800 seminars on troubleshooting refinery process problems to 19,000 engineers and operators. An author of nine textbooks on plant process problems and operations, he holds a BS (1964) from Cooper Union for the Advancement of Science and Art and an MS (1965) from Purdue University, both in chemical engineering.
General introduction
Geared to young and seasoned professionals alike, “Beyond back-to-basics: Process principles and concepts” is a series of articles designed to present a straightforward approach to mastering the principles and concepts all process engineers should be able to apply without the need of a computer.
While simulations and models are useful for examining long-term operational issues, they cannot replace the dimension of human logic and reason required when tackling the array of complex—and sometimes life-threatening—situations that occur in process plants.
Using experiences from the author’s more than 50-year career in the process industry, articles in the series provide approaches to understanding core process concepts in ways that will equip the engineer to walk out of an office, into a plant, and directly resolve process deficiencies via small operational changes or simple retrofits.
This is the ninth article in the series.