Aromatics increase rvp blending value
This article describes a procedure to include the effect of aromatics on rvp blending calculations and presents the pertinent data for these calculations. Refiners that account for this aromatics effect can blend slightly more normal butane into the gasoline pool while meeting the same rvp specification.
In refineries, the rvp blending behavior of pressurizing agents is usually based on standard blending values or on linear blending of the rvp index, which typically is rvp to the power of 1.5. The rvp blending values, however, of pressurizing agents increase with the aromatic content of the gasoline.
Effect of aromatics
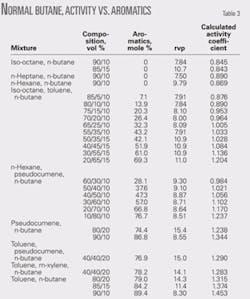
The results of a comprehensive blending study showed a considerable difference in rvp blending values of normal butane with different gasoline components.1 Table 1 shows the results of this study.
The blending value range of 54-66 psi is much greater than errors of 2-3 psi that could be expected with the old rvp test.
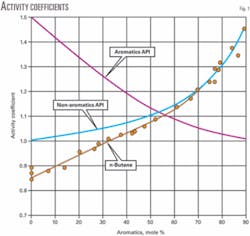
The highest blending values were in the two reformate components. This is consistent with the well-known nonideal blending behavior of nonaromatics with aromatics.2
Luskin and Morris developed a chemical engineering model of the rvp test based on molar blending, accounting for the vapor space in the rvp test and coefficients to correct for nonideal blending.3 The model predicted the rvp of 10 pure hydrocarbon blends of paraffins, olefins, and naphthenes with a standard error of 0.15 psi, using an activity coefficient of 1.0 (ideal solution behavior).
For 18 blends of aromatics with nonaromatics, incorrectly assuming ideal behavior led to an underprediction of rvp of as much as 3.1 psi. Given appropriate activity coefficients, the standard error was only 0.15 psi.
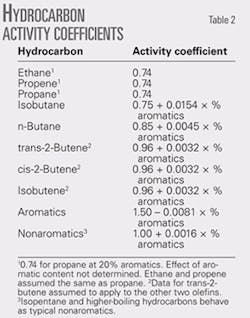
The effects of aromatics on the activity coefficients of normal butane and isobutane are larger than the effects for other nonaromatics. The effects are based on activity coefficients estimated by back-calculation, the chemical engineering model of the rvp test, and data obtained on blends with up to 40% aromatics.
The previously unpublished results in Table 2 show that isobutane is the most responsive to aromatics. Its rvp blending value increases about 1 psi for each percent increase in aromatic content. It is important to minimize the isobutane content of gasoline, and this is particularly true for the higher-aromatic grades.
Table 3 shows the data used to develop the activity coefficient relationship with aromatic content for normal butane. Fig. 1 shows the activity coefficient curve.
Application
To apply the activity coefficients and the chemical engineering model, we use the BLENRVP program4 to calculate rvp for appropriate blends. The BLENDRVP program uses the rvp results to obtain interaction blending equations, which can be applied in the same way as octane and ASTM distillation interaction equations.
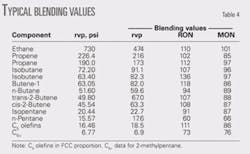
We used pressurizing agent gas chromatograph composition data for 13 different constituents including those that will be present only in trace amounts.
Table 4 lists the constituents and typical octane and rvp blending values.
Refiners can use these data to estimate blending effects of pressurizing agents, but they can obtain better rvp results when accounting for the aromatic content of the gasoline being blended.
Exploiting aromatic effects
Maximizing the use of normal butane in low-aromatic gasoline grades will minimize its rvp blending value, resulting in a slight increase in gasoline volume. Because of other factors present when refiners optimize the compositions of different gasoline grades, this effect will be small, perhaps up to 0.1%.
This upgrading of normal butane over time, however, can become quite profitable.
Acknowledgment
This article is based mainly on work done by Michael M. Luskin at the DuPont Co. Petroleum Laboratory.
References
- Morris, W.E., “The Interaction Approach to Gasoline Blending,” paper AM-75-30, presented at the 1975 NPRA Annual Meeting, San Antonio, Mar. 23-25, 1975.
- API Technical Data Book, 2nd Ed., Washington, DC: American Petroleum Institute, 1970.
- Luskin, M.M., and Morris, W.E., “Reid vapor pressure of Hydrocarbon Mixtures,” presented at the ASTM Symposium, Dallas, Dec. 6, 1973.
- www.gasolineblendingplus.com.
The author
William E. Morris is a consultant in Wilmington, Del., who provides gasoline blending computer programs via his web site, www.gasolineblendingplus.com. He worked for ExxonMobil Corp.’s Bayway, NJ, refinery for 7 years. Then he joined the DuPont Co. petroleum laboratory where he provided technical service for refineries in support of petroleum chemical sales. Morris holds a BS in chemical engineering from the University of Missouri.