Understanding control hazards in fired-heater operations
Norman P. Lieberman
Process Improvement Engineering
Metairie, La.
Young process engineers and operators—and even more tenured ones—find themselves doing their jobs the way they do them because that’s how they’ve been told to do them. In most cases, they’ve been told correctly, while at other times, they find themselves given a set of rules and regulations to follow blindly, never questioning how arbitrary or outdated the rules and regulations might be.
Sometimes a good question can save lives, particularly when it comes to fired-heater operations. To be a good process engineer or operator requires the ability to think on one’s feet and, when in doubt, to ask good questions. This is especially true when instrumentation is involved.
The problem
“I’ve had all the instruments checked several times, and they’re all okay. So, can you please explain to us why sometimes we just can’t control this heater when it’s on auto?” asked Paul, the control-board operator at the Good Hope refinery in Louisiana.
I had walked down to the control room with the process engineer and the shift supervisor, hearing the discussion regarding the H-4 control panel for the visbreaker. The visbreaker heater was running smoothly on automatic control as we talked.
“Tell me, what happens when you lose control of the heater on auto?” I asked.
“The heater can be firing normally, but sometimes when the heater outlet temperature drops—even though the fuel-gas control valve on H-4 opens on auto as expected—the heater outlet temperature continues to fall. Each time this happens, I have to go back to manual control,” Paul explained. “Is it the fuel-gas control system that’s faulty, or should we open up the burner’s air registers when this happens?”
“It’s not that simple. Based on what I’ve heard, it’s probably not a malfunction with instrumentation or the fuel-gas control loop. And I certainly wouldn’t recommend suddenly opening the air registers,” I said.
I thought for a moment on how to best explain what needed to be done, and I remembered it wasn’t so long ago I had the same question from another young process engineer at the refinery.
“Do you all remember Gene, the process engineer that left a year or so ago now?” I asked. “She’d been just as confused as you about the same issue. Let me explain the situation to you the way I explained it to her.”
Learning from experience
Gene had been really excited. She’d just returned from a fired-heater energy conservation seminar in Houston. Full of youthful enthusiasm, she burst into my disordered office.
“Norm! I’m going to save the refinery a huge amount of money by reducing excess oxygen (O2) in our heaters. They taught us at the seminar to target for 3% excess O2 in the heater stack.”
“Gene, you need to be careful,” I said.
“I will, Norm! I’ll get the stack O2 analyzer recalibrated. I’ll tell the operators on the No. 3 pipestill to reduce the flow of combustion air slowly and carefully to the heater. We’re going to save millions and millions of BTUs! I’ll start today.”
“Gene, I don’t want to discourage your sense of adventure, but could you listen to a short story first, before you begin this quest for enlightenment?”
Dangers
“My ex-girlfriend Carole used to say to me, ‘Norm, the reason we get along so well is that I always give you a lot of positive feedback.’ So naturally I formed the impression that positive feedback was a good thing. That’s not always true, though.”
“Let’s say I’m operating a refinery fired heater on automatic temperature control (TRC). This means that the heater’s outlet temperature is automatically adjusting the fuel rate to keep the heater process outlet temperature constant. Let’s say this target temperature is 300˚ C. The combustion air flow is manually regulated by adjusting the secondary air register openings on the fuel burners. Because the outside operator is not available at this moment, however, the flow of combustion air is not being adjusted but remains constant.”
“As the fuel rate increases automatically—because the heater’s process outlet temperature is below 300˚ C.—the amount of O2 in the stack’s flue gas will decrease, which is fine as long as it doesn’t get too low.”
“If the fuel mixes well with the combustion air, having as little as 0.5% O2 in the stack flue gas is okay. If the fuel mixes poorly with the combustion air, however, having as much as 5% O2 in the stack flue gas may be too little.”
“What do you mean by too little oxygen in the flue gas, Norm?” Gene asked.
“What I mean is that, if you reduce the flow of air a little and keep the fuel rate steady, the heat of combustion of the fuel will decrease. Instead of all the carbon in the fuel burning to CO2, some of the fuel’s carbon will burn into carbon monoxide (CO). Burning carbon into CO releases a lot less heat than burning carbon into CO2.”
“How well the fuel and air mix depends on burner efficiency, which is very variable, depending on the design and condition of a burner. Let’s assume burner efficiency is such that an O2 content of 2% in the stack flue gas is just about right (meaning that having 1.5% O2 in the stack flue gas decreases the carbon burned into CO2 and increases carbon burned into CO). The result of adding more fuel without more air causes the heat generated in the firebox to diminish.”
This wouldn’t be a big deal if the fuel was adjusted manually. If the fuel adjustment is on TRC, however, then the reduction in heat generation by adding more fuel will cause the heater process outlet temperature to drop below 300˚ C. and cause the fuel-flow rate (but not the air rate) to increase. The increased fuel-flow rate drives the heater outlet process temperature lower still because of increased CO in the stack flue gas, which causes the fuel-flow rate to continuously increase as the heater process outlet temperature further decreases. The heater is now caught up in a positive feedback loop.
“You see, quite unlike that relationship with Carole all those years ago, a positive feedback loop isn’t a good thing for a heater because, with time:
• Black smoke will be emitted from the heater stack.
• The heater firebox will become hazy with long, licking, yellow, smoky flames.
• Fire will eventually race out the top of the stack.
• The heater outlet temperature will continue to diminish, while the fuel-gas rate increases exponentially.
• If the outside operator now intervenes by manually increasing air flow suddenly through the burners, the heater will blow up, thus resolving permanently all the outside operator’s problems (likely killing him, and perhaps others, in the blast).”
“What the panel board operator should have done first is switch to the manual mode of control for the fuel-flow rate and then back it down until the process heater outlet temperature starts dropping. Only then is it safe for the outside operator to increase the combustion air flow rate manually through the burners’ air registers.”
“My relationship with Carole ended in the same way: it kind of blew up. I think the positive feedback loop she referred to consisted of me buying her progressively more expensive presents and Carole trying to put up with my introverted personality.”
“It happens,” Gene said.
“Yes, it does,” I said, and continued with the explanation.
Variable wind
“Wind blowing across the top of a heater stack will increase draft and draw more combustion air into the firebox. If the wind suddenly diminishes, the flow of air will momentarily drop. If the heater is running too close to its optimum combustion air rate, the wind will cause it to drop briefly below this optimum air rate. The heater outlet temperature will then drop, and if the heater is operating on TRC, the following sequence will occur:
• The fuel flow rate will increase.
• The heater outlet temperature will further decrease.
• The fuel flow rate will increase even more.”
“The heater will now be caught in a potentially dangerous positive feedback loop, and suddenly opening the air registers to break the loop may blow up the heater firebox.”
Absolute combustion
“I call the air-to-fuel ratio that maximizes the heat generated or released by burning a pound of fuel the point of absolute combustion. Decreasing the air-to-fuel ratio below this point reduces the temperature of the burning fuel. The amount of CO, light alcohols, ketones, and other products of partial oxidation of fuel will increase exponentially. The operators will find their heater cannot be run on TRC and that the fuel rate must be controlled manually. Operating a fired heater below the point of absolute combustion also wastes energy.”
“If the fuel is a liquid, heavy industrial fuel oil (e.g., Bunker C) used on ships and power plants, dropping the air-to-fuel ratio below this point will cause the color of the flue gas emitted from the heater’s stack to go black. If the fuel is a gas, the color of the flue gas from the stack when operating below the point of absolute combustion may turn pale yellow or slightly orange. If the fuel was hydrogen, the flue gas color will stay clear.”
“The important concept to remember is that the point of absolute combustion will vary with time depending on the air-fuel mixing efficiency of the burner. Typically, I have seen it vary from 0.5% to over 6.0% excess O2 in the stack’s flue gas.”
To further illustrate the concept, I shared two more stories with Gene related to me by a couple of plant operators.
Visbreaker heater outlet temperature
“When I was teaching a seminar for Chevron Corp. in its former Port Arthur refinery quite a few years ago, a young operator said he had a question.”
“‘Mr. Norm, my visbreaker unit is really old. The heater is old, the controls are old, and we run the heater fuel on manual because the controls are so old.’”
“What’s the question, Tommy?” I asked.
“It’s that last week, by accident, I turned on our third forced-draft air blower, and the heater outlet jumped to 720˚ F. from 680˚ F., with the same fuel flow. I never moved the fuel flow. That’s what happened. What do you make of that?” asked Tommy.
“When you saw the heater outlet temperature jump up like that, what did you do, Tommy?”
“Well, of course, I shut the blower down, Mr. Norm!”
“Tommy, you should have cut the fuel rate back and left that third blower on. Obviously, you must have been running the heater in an air-deficient manner before you turned on the third air blower,” I explained.
“But Mr. Norm, we had more than 3% O2 on our stack-gas analyzer with just two blowers on. Our target is 3% O2. We were given this target by Mr. Henry himself, the plant manager. With the three blowers on, our excess O2 was more than 6% in the stack! I’m really confused, Mr. Norm. Should I run at 3% or 6% O2 in the stack?”
“What are you trying to accomplish, Tommy: saving fuel or saving air? You must think about what you’re doing. Most likely, you have a lot of tramp air leaks in that visbreaker heater’s convective section.”
“Well, maybe the 6% O2 would be better to save fuel, but my main objective is to get paid and keep my job. Mr. Henry set the target at 3% in the stack. If we run the heater above that target, then it’s time off without pay, and I might get fired. Then what? Go back to hunting gators in the swamp and collecting Spanish moss? I think I’d like better to keep on the right side of Mr. Henry and keep my job at the refinery.”
Boiler-steam generation rate
“And then there’s the story of George, an old utility plant operator at Holly Frontier Corp.’s refinery in Salt Lake City, Utah.”
“Mr. Lieberman,” George said one day. “I’ve got a target of 2-3% O2 in the stack of my 650-psig steam boiler. I run at a constant fuel rate. Mr. Bloominthale, our vice-president, set that O2 target himself!”
“George, what’s your point?” I asked.
“Mr. Lieberman, when I increase air to 4% O2, steam production increases. That’s my point!” George hollered.
“This is a true story, Gene, except for the vice-president’s name. The story about the increase in heater outlet temperature with the use of the third air blower is also true. So you have to ask yourself, what insanity has seized our industry for stories like these to be so universal?”
O2 analyzer placement
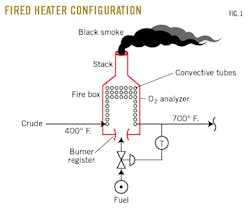
“Gene, take a look at this schematic of a fired heater (Fig. 1). See that I’ve shown the O2 analyzer below the bottom row of convective tubes. If the O2 analyzer is in the stack, as many of them are (Fig. 2), then tramp-air leaks in the convective section can cause the analyzer to read higher than the O2 in the firebox, where the combustion is taking place,” I explained.
Gene nodded in agreement, so I continued my explanation.
“If the leaks are large, then closing the air registers at the burners can increase the O2 reading in the stack. This happens because restricting the air flow through the registers increases draft (i.e., reduces the pressure in the firebox). This creates more tramp-air flow into the convective section and influences the O2 analyzer if the analyzer is in the stack.”
I explained to Gene she needn’t worry about this, though, because on our No.3 pipe still, the analyzer was in the proper place (Fig. 1).
Takeaways
Improving air-fuel mixing efficiency reduces the amount of excess air needed to maximize heat release from a given amount of fuel. If a heater is operated on automatic temperature control, which is typically the case, then falling below this optimum amount of combustion air will cause the fuel rate to increase while the heater outlet temperature declines. Plant operators refer to this as “flooding the firebox with fuel” or “stalling the firebox.”
A sudden increase to the proper amount of air flow by opening the burner secondary air registers and-or rapidly opening the stack damper can cause—and has caused—process heaters to explode, causing fatalities.
I shared with Gene a rather similar incident in which I was involved in 1980 during the strike at the former Amoco refinery in Texas City, Tex., when I’d inadvertently closed the air register of the sulfur plant incinerator. Seeing that the incinerator temperature was rapidly dropping, I realized my mistake and—without thinking—rapidly opened the air register. The resulting “thump” knocked me off my feet.
“Of course, Gene, that was a long time ago, and since I learn from my mistakes, I’m much smarter now.”
Gene was lost in thought. I suspected she had stopped listening to me some time ago.
“You know, Norman, you’re right! I’ve got a good idea. What I’ll do is put the fuel on manual first. Then, I’ll try increasing and decreasing the combustion air flow through the burner air registers. I’ll find out what amount of excess O2 in the stack maximizes the heater outlet temperature with a fixed amount of fuel, and then I’ll use this as a target for the point of absolute combustion. What do you think, Norm?”
“Excellent plan, Gene,” I said. “But always run somewhat above your target O2 and recheck your target O2 periodically. Remember that the target O2 is a variable dependent on all these factors that we’re discussing. What you’re planning is pretty much the same as what I saw the operators controlling Murphy Oil’s crude heater do in New Orleans a few years back, and I recall that it was working quite well. The point of absolute combustion for that heater was an ideal 0.56% O2 in the firebox.”
Lesson learned
Back in the central control room at Good Hope, Paul looked a lot more relaxed.
“So that’s how I explained this to Gene, Paul,” I said. “Does that help you?”
“Well, Norm, I can see now that opening air registers when a heater is caught up in a positive feedback loop is dangerous. The safe thing to do is to get above the point of absolute combustion by manually reducing the fuel-gas flow before increasing the combustion-air flow rate. Thanks for sharing that.”
“You got it, Paul,” I said.
The author
Norman P. Lieberman ([email protected]) is a chemical engineer with more than 50-years’ experience in process plant operation, design, and field troubleshooting. He previously served as operations supervisor, technical service manager, and plant manager at US refineries until 1985. An independent consultant, he also has taught 800 seminars on troubleshooting refinery process problems to 19,000 engineers and operators. An author of nine textbooks on plant process problems and operations, he holds a BS (1964) from Cooper Union for the Advancement of Science and Art and an MS (1965) from Purdue University, both in chemical engineering.