Refiners discuss trends in hydroprocessing
During the 2017 American Fuel and Petrochemical Manufacturers Operations & Process Technology Summit (formerly Q&A and Technology Forum), Oct. 2-4, 2017, US domestic and international refiners discussed trends in hydroprocessing operations, with an extended focus on topics of safety, hydrogen plant operation, and sulfur issues with hydrotreating-hydrocracking catalysts.
This annual meeting addresses real problems and issues refiners face in their plants and provides an opportunity for members to sort through potential solutions in a discussion with panelists and other attendees.
This is the first of three installments based on edited transcripts from the 2017 event. Part 2 in the series (OGJ, Oct. 1, 2018) will highlight discussion surrounding gasoline processes, while the final installment (OGJ, Nov. 5, 2018) will focus on fluid catalytic cracking (FCC).
The session included a six-person panel comprised of industry experts from refining companies and other technology specialists responding to selected questions and then engaging attendees in discussion of the relevant issues (see accompanying box).
The only disclaimer for panelists and attendees was that they discuss their own experiences, their own views, and the views of their companies. What has worked for them in their plants or refineries might not be applicable to every situation, but it can provide sound guidelines for what would work to address specific issues.
Safety
What are some of your typical examples of integrity operating window (IOW) types which are specific to hydroprocessing? What technique do you use to determine severity level of IOW alarms? How do you coordinate operator response to IOW alarms?
Aggus At Becht Engineering, we are now working with clients very frequently to develop integrity operating windows. So, I want to begin by saying that although the FCC guys will argue with my opinion, I believe the subject of IOWs is unique in refining because you have everything you wouldn’t want for mechanical integrity. You have high temperature, high pressure, a process that can run away, and high concentrations of both hydrogen sulfide and hydrogen. You also put a little water in the process just for fun.
I find that within a lot of the sites where we work, the definition of the IOW varies. For some of the refineries, the IOWs are synonymous with the safe operating limits (SOLs). These are limits that require immediate operator response. Parameters—such as operating temperature limits, as we are discussing—can be considered IOWs in those cases. Other refineries are in a unique category where the critical limit would be something that requires 24-48 hr for a response. A downtime informational limit is something that could have a long-term (months or years) type of impact. The value of these informational alarms is often up for debate. For the guy sitting at the console who must deal with the alarm, it’s sort of a nuisance. It’s better monitored by the engineers via their own monitoring tools, which is my take as well.
HollyFrontier Corp. subsidiary HollyFrontier Navajo Refining LLC operates the 100,000-b/sd Navajo refinery on a 561-acre site in Artesia, NM. It runs in conjunction with a crude distillation unit and associated vacuum distillation units in Lovington, NM, about 65 miles east of Artesia. Located near the Permian basin, the refinery processes sour crude oils purchased from independent producers in southeastern New Mexico and West Texas into light products, including gasoline, diesel, and jet fuel. Photo from HollyFrontier.
My recommendation is for sites to maintain commonality between SOLs and IOWs, if only just to eliminate confusion between operations and engineering personnel. In that case, your critical IOW alarms would be the ones associated with exceeding maximum-allowable workable pressures, including those that could induce cracking mechanisms, like getting in a 50º F. or a 25° F. approach to your Nelson curve for high-temperature hydrogen attack or anything that could rapidly foul your process equipment; for example, total loss of washwater. By this methodology, standard alarms would be the 1-week window ones. For example, a typical standard alarm would be low-washwater rate (e.g., less than 10% free water remaining after injection into the reactor effluent stream). Reactor heater outlet temperatures that are getting close to a high-temperature sulfidation limit or separator temperatures that are approaching your desublimation temperatures would be standard alarms also. Another example, in the case of gas oil hydrotreaters, would be hot-feed temperature approaching naphthenic acid-corrosion temperature: about 450° F. or so.
For many sites, setting the severity level of the alarms is a collaborative effort. You get the process engineer and the fixed-equipment engineer involved, as well as the ops team. Then, if a site has a reliability engineer, he or she tends to lead the team. Many refineries—although not all—are managing the IOWs through the management-of-change (MOC) process. I think that’s a great idea because not only do you get those groups involved, you would then also be involving process safety personnel, as well as have environmental personnel bringing a little insight to the effort.
The flow-of-operator response process is typically coordinated by the board operator at most refineries we have come across. So, he or she as the first point of contact with the alarm and the required response will typically receive it via the distributed-control system alarm management system. The board operator is the first set of eyes, so it makes sense for that person to respond. Some sites are now proceduralizing the responses to these alarms. I don’t know the efficacy of that approach. Often, excursion is best dealt with using operator judgment rather than a controlled procedure. So, for a lot of refineries, we also find that their alarm management system can text, page, and e-mail both the process engineer down on the bottom and your fixed-equipment engineer up on the top. So, almost 10% of the time, operations response will be coordinated through the console supervisor. It’s best, however, to have a notification system so the engineers are aware of the event. I recommend using that system to contact your engineers.
Johnson Motiva’s approach to integrating operating windows is joined at the hip with the safe operating limits where we have multiple layers of data we are monitoring. In the beginning, we set up these limits into different layers to manage corrosion limits, safe operating limits, and any other pertinent information available. All the data is managed through an MOC work process. Process engineers are interfacing and performing alarm rationalization as part of their daily routine work with console operators to ensure that these layers we have are appropriately set, abided by, and responded to accordingly. Whether it’s with procedure or the electronic system that’s monitoring parameters, all of this is performed daily. Motiva’s approach is a multilayer, multidiscipline approach.
Hydroprocessing
How are developments in hydroprocessing catalyst adjusting to changes in feedstock quality? Are the new developments able to cope with and provide high activity with varying feedstock severity?
Al-Fudhail As we know, in feed to the hydrocrackers, hydroprocessing and hydrocracking are becoming more and more refractive and difficult. This challenge is leading to the need to increase catalyst-to-hydrogenation treatment capabilities. Of course, with the coker gas oil and the asphalted oil, hydrofractive material increases in the fraction of the feed. Operating the light coker, as well as units with very low recycle, to reduce coke nick, heavy coker gas oil becomes highly refractive.
There are basically two ways of combating that new, elevated refraction. One is with catalyst development. Another is to adjust the catalyst reactor’s loading. By means of layering your catalyst load, you will be processing Type I treating catalyst and Type II, as well as immediately placing into activity those catalysts, like light-cracking catalyst used to denitrify and saturate the feed. Of course, Type II treating catalyst has a much higher hydrogenation function. This trend of using intermediate cracking catalyst to finish the pretreatment is gaining ground as opposed to using nearly amorphous silica and alumina catalyst, as was done in the past.
Modern cracking catalyst also uses a mix of traditional Y zeolite along with beta for optimum cetane with low cold-flow characteristics. This technique optimizes hydrogen consumption and reduces the cold-flow properties for the distillate while maintaining optimal cetane index.
Now when processing vacuum gas oil (VGO) from ebullated-bed hydrocracking that is extremely aromatic, there will be a bulk-metal hydrotreating catalyst sandwiched between Type I and Type II. A treating catalyst is used to ensure that VGO is treated to meet feed quality for the FCC or second-stage catalyst in a maximum convergent unit. In such units, processing ebullated VGO second-stage catalyst requires high-hydrogenation capabilities, which leads to the increased use of NovoMetal catalyst.
Johnson In Motiva, we’ve seen advances in technology with the catalyst generations gaining more activity not only in VGO service, but also in distillates.
We’ve discovered that when evaluating a catalyst selection, depending on the unit configuration, we can unlock potential with not only catalyst, but also with hardware adjustments to extract more value from the catalyst, in terms of cycle length and sustaining yield over the cycle. In short, we haven’t seen the issues where the catalyst companies are not keeping up with the changing feedstocks with which we are dealing.
Schoellkopf I’ll basically echo much of what you’ve already heard. We’re seeing more and more difficult molecules that need to be processed. Not only are there complicated molecules, but they’re making up a higher percentage of the feed; therefore, it takes some innovative approaches to the catalyst-system design to resolve this challenge. The conventional methods are no longer necessarily sufficient. More of a good thing is not necessarily the optimal solution, either. That is, you don’t necessarily want to go for high hydrodenitrification (HDN) or high alumina for HDN processing when you’re also processing some other highly reactive feeds (e.g., heavy coker gas oils). So, we generally just take feed from the refiner and tailor our loads based on what the refiner wants in the products.
Pappal I think there are a couple of universal rules in hydroprocessing or hydrocracking. You can never have too much reactor volume, and you can never have too much catalyst activity. As catalysts improve, refiners will take advantage of catalyst activity and reactor volume.
Kirk I just want to say that it’s key for catalyst suppliers to keep up with the increasing demand on activity by making sure we have a fundamental understanding of the surface science. That way, you’ll be able to optimize the different parameters of the catalyst with respect to pore-size distribution, pore volume of the catalyst, and catalyst surface area, such that you can tailor those parameters to the type of service for which you’re designing the catalyst, as well as to the severity of the feed that you’re treating.
Also, with the increase in severity of feed, you’ll see an increase in the amount of metals on the catalyst. So, you need a good metals-trap catalyst. We’re seeing a lot of arsenic and silicon, even in straight-run components that are typically supposed to be the easier-to-treat streams. You can have all the activity in the world in your main bed catalyst, but if you don’t also have a good guard bed to protect that high-hydrodesulfurization (HDS)/HDN activity, the catalyst load will not have very good stability.
Zink Catalyst development has focused on this very issue in its development of several of the highest-activity hydrotreating catalysts in the Unity hydrotreating catalyst portfolio. Catalyst support is engineered to facilitate less diffusion resistance in the same extrudate size-shape class relative to its peers, but with improved interaction between the active phase and the support, leading to greater relative effectiveness for HDN-HDS-hydrogenation and for uptake of metals like vanadium and nickel. This feature allows for the refiner to sometimes process heavier, more difficult feeds during the cycle. Bundling this with a relative minority fraction of hydrocracking catalyst and-or aromatics-saturation catalyst downstream can be applied to target specific yields and qualities of finished products. Further advancements in alumina preparation to effect greater relative surface area have led to improved silica uptake. Combining this feature with improved metals-finishing techniques allows for greater relative HDS-HDN activity compared to the former generation.
Hydrogen
What are the variations of target efficiency that can be achieved in hydrogen plant operation? What are the operational factors that impact efficiency?
Long Hydrogen plants have several areas to target when it comes to efficiency. There are several factors that contribute to energy efficiency, and all process variables vary greatly from plant to plant.
I’m going to start with the pressure-swing adsorber (PSA). The main target-production efficiency of a hydrogen plant is the PSA efficiency. This is calculated as a ratio of PSA product hydrogen to inlet PSA hydrogen. A PSA efficiency greater than 85% is adequate. Another ratio to consider is efficiency of conversion, which is the unit feedstock-to-hydrogen production ratio. This efficiency of conversion has an impact on total plant operations. Typical efficiency of conversion is 2.1-2.4, and variations in the plant average value can indicate operational upset. Operational impacts on PSA efficiency include valve-switching failures and PSA feed gas. As carbon monoxide (CO) concentrations increase in feed gas, the efficiency of the PSA is reduced. Another way to measure energy efficiency is by evaluating the energy conserved per unit of hydrogen produced.
Now we move on to the reformer. The factors that can impact energy efficiency in a steam methane reformer (SMR) indicate catalyst activity, burner operations, heat lost to atmosphere, furnace operation, heating values in btu, tube life, shift equilibrium, or steam-to-carbon ratio, and potentially ambient temperature. A direct-monitored target for SMR consists of methane slip and outlet target temperature, which varies from plant to plant. Methane slip consists of 1.5-5.0 dry mol % and impacts heating values in terms of btu. Methane slip is controlled in the reformer by shifting the equilibrium or manipulating the steam-to-carbon ratio and SMR outlet temperature. Typical steam-to-carbon ratio is 2.0-3.5, but this ratio can greatly vary from plant to plant.
If the reformer reaction equilibrium was shifted to increase hydrogen make, steam, or temperature, this modification will reduce methane content in the PSA offgas in the reformer heater. As btu values of the offgas decrease, the secondary burners may have to be fired harder, which will require an increased amount of purchased natural gas. Increasing methane slip correlates to an increase of heating efficiency and potentially an increase of reformer tube life. Increasing methane slip is achieved by decreasing the steam-to-carbon ratio or decreasing reformer outlet temperature. To increase hydrogen production, the steam-to-carbon ratio can be increased; however, impacts to heating value and tube life should be considered. It should also be noted that operating at higher reformer temperatures will directly decrease tube life and catalyst life.
In the high-temperature shift converter, the factors that impact energy efficiency in the shift converter are the inlet temperature and catalyst activity. The shift converter should target constant inlet temperatures, as temperature swings impact catalyst activity.
The exotherm across the shift converter should be monitored, as well as CO slip. Inlet temperature can be increased to maintain constant exotherm as catalyst deactivates. The local startup temperature differential is 100º F. and a target startup run temperature below normal. It’s common to have temperature step changes that occur every 6 months. If a plant is short on hydrogen, the inlet temperature can be increased to promote CO conversion. The target shift converter CO slip consists of 1.5-3.0 dry mol % and indicates catalyst deactivation. This percentage depends on each plant, as well as start-of-run conditions. The operational factors that impact efficiency of CO slip are inlet temperature and the steam-to-carbon ratio. CO slip can be decreased by increasing inlet temperature or increasing the steam-to-carbon ratio. The temperature differentials will be indicated by catalyst activity. Inlet temperature can also be increased to achieve the target temperature differential, which changes from plant to plant.
Lastly comes the sulfur guard. Front-end sulfur removal has an impact on catalyst efficiency in the hydrogen plant. The sulfur component removed is hydrogen sulfide (H2S) and variations of mercaptans. The desulfurization previously consisted of activated carbon at ambient temperatures. Desulfurizers have used several types of media, from zinc oxide to carbon beds. The media type can impact efficiency, depending on the plant design and temperature parameters.
Aggus All I’ll add about efficiency is that a lot of the people who designed the plant—CB&I, in particular—use a plant efficiency term. It is not just the amount of natural gas you convert to hydrogen; you must throw the steam in there as well. I don’t know how many hydrogen plant unit engineers we have here, but to those who are in the audience, I want to say that it’s a good exercise to add this efficiency calculation to your daily monitoring. You want to take the amount of feed on a heating-value basis, the fuel that is going into the furnace, and then the difference between your energy and boiler feed water to steam, and then divide that by the hydrogen product. If you monitor those values every day, you’ll be doing a good job of taking care of your SMR. If only it was that simple, right?
The heavy hitter, as far as energy usage and efficiency in your SMR, is the furnace. It’s just like any furnace: You want to be able to monitor your excess oxygen and excess air. So, try to maintain 2.5-3.0% excess O2 in the furnace. What also really affects the efficiency of hydrogen recovery, as Sarah said, is the PSA. The unit temperature is easy to monitor. Make sure you’re staying below 110º F. It’s your PSA. You’ll have best absorption efficiency if you do that. Then if you’re really nice to your process control engineers, you can also play with the cycle time on the PSA. So, longer absorption times will give you less hydrogen loss and blowdown repressurization and should increase your recovery.
Ludwig I’ll just say that the typical minimum for steam-to-carbon ratio is 2.8. I think the panelist said 2.0. We think you’d run into real problems running at 2.0. The other important point is pressure: the lower the pressure you can run on the outlet of the SMR, the better the equilibrium. That is usually a design consideration, however, and not something that the operator can do with an existing SMR.
Chlapik We put a lengthy answer in the Answer Book, and I want to add two comments about efficiency now. We’re hearing that a lot more hydrogen plant operators want to address and improve on efficiency. As you start to change some of these variables, monitoring your unit will become even more important. The steam methane reformer, as has been said by the panelists, is a critical part of that hydrogen production unit. We’ve been working with Daily Thermetrics over the last decade and have developed and established the application of their CatTracker thermometry for in-tube reformer thermometry. CatTracker for reformers gives you a “sight glass” in that reformer, with respect to the reactions that are going on in the tube. When running the reformer at these more efficient conditions, the operator can see the quick response of operating changes as they are made. This enables the operator to maintain reliability at these new conditions, which is so important in hydrogen production.
My other comment is that there are operators who are trying to look at low-capital ways to address efficiency and production. Usually when you start looking at improving efficiency at low capital, this limits the number of options. Johnson Matthey has a step-out reforming technology in our CATACELJMSSR (SSR), a stackable-structure reactor that replaces the catalyst pellets in the reformer tube. SSR enables an operator to make a step change in efficiency through improved heat transfer, activity, and pressure drop in the reformer tube, allowing the same production with 5-10% less firing or 5-10% more production before plant modifications.
Sulfur
Under what conditions will you strip sulfur from hydrotreating-hydrocracking catalysts?
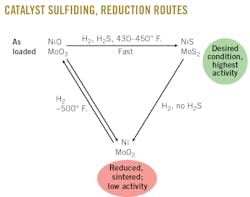
Schoellkopf Generally, the catalyst is above 500° F. In the presence of hydrogen without the presence of H2S, the rate in which the sulfur can be stripped off the metal sulfide is very quick. Note that the rate of reduction reaction is determined by the hydrogen partial pressure, temperature, and time, with temperature having the strongest impact. There are a multitude of scenarios that can cause H2S to not be present in the reactor and, therefore, strip sulfur from the catalyst. Typically, during sulfiding, your sulfiding-agent pump trips, which is the origin of your H2S, or the H2S is purged for the open-valve misalignment. Another possibility is that the aiming system is accidentally lined up. Depending on during which stage of the startup this occurs, you will have a relatively minor inconvenience or a more complicated issue. In the case where dimethyl disulfide is lost and you’re above 500° F. and at pressure, the reduction reaction will occur quickly. The same is true for mechanical failures of equipment such as recycle compressors or feed pump trips.
The reversible sulfiding reaction must be conducted outside of the reactor (see figure). So, once you’ve stripped the sulfides off the catalyst, you will basically have to start all over again. If H2S is lost for any reason during the startup, ART recommends immediately beginning to reduce the temperature to get the catalyst below 350° F. If possible, continue circulating hydrogen until the unit can restore the operating conditions. If the reason H2S was lost is expected to continue longer than 24 hr, then cooling to below 250° F. is recommended to minimize any reduction opportunities. You just don’t want to have any hot spots within the reactor.
Hydrogen stripping can also be very useful for removing soft coke and restoring some lost catalyst activity. That process, however, can also lead to removal of sulfur from the catalyst. A minimum of a 1,000 ppm H2S is recommended to be maintained within the recycle loop during the hydrogen-stripping procedure to avoid any accidental reduction. We also suggest working with your catalyst supplier for any additional specific guidelines.
For a temporary shutdown (defined as < 24 hr or up to 48 hr), it’s preferred that you shut down the scrubbing device, of course, and purge with nitrogen. Depending on the H2S content, however, recycle gas can be at some acceptable temperatures to hold the unit: the cooler, the better. You continue circulating hydrogen, assuming you have plenty of H2S in the recycle loop: 1,000 ppm or better, though 2,000 ppm or better is recommended. Then, of course, it’s always recommended to work with your catalyst vendor for any specific unit guidelines and anything specific to your unit.
Long At Navajo of HollyFrontier, we haven’t had any experience in recent history of stripping sulfur from the catalyst. Most incidental or inadvertent stripping of sulfur from hydrotreating catalyst occurs during startup of the unit. We mitigate that potential within our startup procedures, which state that temperatures aren’t to exceed 400° F. during the hydrogen once-through heat-up purge before introducing the feed. The biggest impact would be on catalyst-run length. The highest risk arises if all sulfur is removed and the catalyst is stripped to base metal. At that point, the catalyst is completely deactivated.
Al-Fudhail Just to add to what has been mentioned already, pilot-plant testing has verified that cobalt-based catalysts require higher H2S concentrations than nickel to remain sulfided. The tendency to reduce will be higher for a clean catalyst system in startup cycle or even clean-feed service.
Floor question: As you said, a common catalyst requires a higher H2S concentration. Do you have any idea of how much higher? Is it 20% or 50% higher?
Al-Fudhail I don’t really have a quantitative number. You’d typically want it to be in the 100-500 ppm range. Imagine a rounded-up higher number–500 ppm or close to 1,000 ppm–of H2S concentration to keep it in a sulfided state.
Robledo I think the point of the question was that we all understand where you’re in oxidic state. You’re going to reduce the catalyst very fast, right? Often, the question comes up when you’re up in operation and have been running for a year or two. My question to the audience is: How many of you have run a hydrotreater at 600° F. with no H2S present, and for how long of a window-activity penalty?
Aggus Can I answer that for those who don’t wish to be identified? I do know of one instance, during a hot-hydrogen strip, where it hadn’t been previously carried out on the unit. The procedure used previously had been employed for another unit without an amine absorber in the recycle circuit. In this case, the H2S absorber is left in operation and the temperature run up to 700° F; so, 8 hr and no loss of activity. At least the way that it was monitored against the budget curve, for the remainder of that run, there was no decrease in overall catalyst activity. The amount of energy required to get stripping of sulfur done, therefore, may be higher than some may think.
Moreland We had a similar incident where we had a unit on standby circulation. Per procedure, we cooled the reactor down to 450° F. The amine scrubber was off, so we monitored the H2S build in the circulating gas. It had built up to between 50-100 ppm, at which point we have sufficient concentration of H2S to keep the catalyst sulfided. So, I agree with what was said: There is no activity effect, but sulfur will come off the catalyst. I’ll say that some of the sulfur that comes off the catalyst can be put back on as soon as feed is introduced without much of an activity penalty.
Al-Fudhail I’ve worked with a hydrocracker, and my experience was that it was quite a troublesome unit. We had a lot of shutdowns and many ups and downs. Really, there were times when we ran the recycle gas with fresh catalyst after a shutdown and after a purge. There isn’t really much H2S in the system. Once you get the feed into the unit, whatever sulfur that has come off will soon come back and be sulfided in the catalyst. Really, not much penalty or any reduction in activity has been noticed.
Now coming back to when we’re done with the unit or getting ready to shut it down for a catalyst changeout, we do the hydrogen hot strip. And boy, that catalyst system still emits sulfur H2S, even after we’ve done the 750° F. hydrogen strip with no H2S, clean hydrogen. You take it offline and try to slip that blind to get out a lot of H2S from that reactor. So, I don’t know, because I’ve never experienced sulfur stripping out of a catalyst system.
Gripka I’m going to respond to Brant’s comment and the other comments made after his. There will be some H2S coming off because you have coke on the catalyst. There’s some sulfur inside that coke which you put on the catalyst, so a lot of it depends on how heavy the feed is, how long you have operated, etc. I think the recommendation will still be to err on the safest side, like Lyle suggested. If you’re doing a hydrogen strip, 1,000 ppm H2S is a good place to be. Like Andy said, it’ll build up some, so 1,000 ppm H2S is essentially a safety margin for you to operate under.
The panel
Brant Aggus, process engineering-safety manager, Becht Engineering Co. Inc.
Noaman Al-Fudhail, superintendent, Riyadh refinery engineering, Saudi Aramco
Errol Johnson, process engineering team lead, Motiva Enterprises LLC
Sarah Long, process engineer, Navajo refinery, HollyFrontier Corp.
David Pappal, technology advisor, Valero Energy Corp.
Lyle Schoellkopf, technical services manager, Advanced Refining Technologies LLC (ATR)
The respondents
Ken Chlapik, Johnson Matthey PLC
Patrick Gripka, Criterion Catalysts & Technologies LP
Travis Kirk, Haldor Topsoe Inc.
Lewis Ludwig, UNICAT Catalyst Technologies Inc.
Andrew Moreland, Valero Energy Corp.
Sergio Robledo, Delek US Holdings Inc.
Steve Zink, Honeywell UOP LLC