BEYOND BACK-TO-BASICS: PROCESS PRINCIPLES AND CONCEPTS-2: Understanding reflux in distillation towers
Norman P. Lieberman
Process Improvement Engineering
Metairie, La.
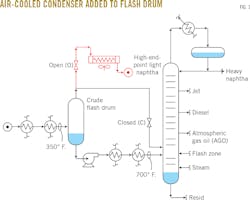
Following installation of a new air-cooled condenser as part of its crude distillation unit's preflash tower overhead, a US Midwestern refinery began producing off-spec light naphtha that led to catalyst coking in a downstream naphtha reformer.
Using the basic process principle of a partial condenser applied years earlier to revamp an ancient Pisco distillation still in Peru, the author recommended simple modifications to route reflux from the new condenser back to the flash drum's overhead, resulting in a low-cost fix to the refinery's problem.
High-endpoint naphtha
John Henry, operations manager of a 30,000-b/sd refinery in southern Illinois, was having issues following recent improvements made to increase processing rates at his crude unit.
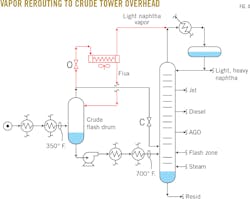
"Norman," John said. "The project you suggested last year has been a disappointment. I repiped the crude flash drum vapors to the new air-cooled condenser we installed in the October turnaround (Fig. 1). As planned, diverting those cool, 350° F. vapors out of the flash zone has increased the flash-zone temperature in the crude unit by 30° F. This unloaded the crude tower trays, so we've been able to increase the crude rate by 3,000 b/sd."
"John, that all sounds good. What's the trouble?"
"My trouble is that the light naphtha from the new flash drum air cooler's vapor has a 420° F. endpoint, and that long, heavy tail is coking up my naphtha reformer catalyst. I need to get the ASTM D-86 distillation endpoint to a maximum temperature of 380° F."
Well-acquainted with the unit following our collaboration on the project, I thought for a moment and decided to tell John a story to illustrate the idea I had in mind to solve his high-endpoint naphtha problem.
Lessons along the way
A few years earlier during a visit to Peru, I had an opportunity to tour a very old pisco distillery in the remote countryside. Pisco is the main ingredient of a Pisco Sour, the country's national drink.
Located on the road to the ruins of Machu Picchu, the distillery had what appeared to be hundreds of clay jars or pots piled in one corner of the site while huge stacks of firewood loomed in another area.
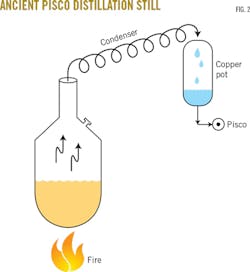
The still itself consisted of an 8-ft wide by 5-ft high copper pot set on a red-brick structure above a brick-lined chamber into which firewood was fed. A long coil of copper tubing condensed the pisco vapors from the still and drained the condensed vapors into a second man-sized vertical copper pot elevated about 16 ft above the top of the still (Fig. 2).
I was introduced to Juan Ramirez, the distillery's lead operator. A middle-aged man of Indian extraction and very knowledgeable in regard to the plant, Juan explained the distillery measured the alcohol content of pisco (25-30%) with a float. If the pisco was too weak, the line on the float would be above the liquid level in their test glass, indicating a too-high water content (i.e., the pisco's density was too high).
"So Juan, if the pisco is too weak, what do you do to bring it up to 30% alcohol?" I asked.
"Maybe next batch, we don't cook it so hard; use less wood so juice cooks slower and colder. Mucho caliente makes pisco too weak. Or maybe, Senor Norm, I put weak pisco back into the still and heat it up a second time, because its second trip through our still will make the pisco very strong," Juan explained.
For me, this is what process engineering is all about. It's that interface where I meet the plant operator, and we resolve our understanding of a problem using applied technology.
Albert Einstein once said that America's greatness comes not from freedom, equality, or democracy but from the fact that it is the foremost land of applied technology.
Vapor-pressure limitations
I then asked Juan why he would sometimes cook the juice too hard if it vaporizes too much water with the alcohol and makes weak pisco.
"Because, Senor Norm, we make more money by producing more pisco."
And that, in a nutshell, is the original problem of chemical and process engineering: the balance between product recovery and product purity. To keep his product on-spec at a minimum of 30% alcohol, Juan had to reduce product recovery by burning less firewood underneath the still.
The problem is that alcohol has a large vapor pressure while water has a smaller vapor pressure. When the still is fired harder, the vapor pressure of the water increases faster than the vaporization rate of the alcohol from the fermented juice. So while the amount of juice vaporized increases, the amount of water vaporized increases faster because the vapor pressure of the water is increasing at the hotter temperature in the still.
Reducing the still temperature reduces water vaporization, but also reduces pisco production. That's the price Juan pays, however, for making the pisco stronger to meet his alcohol spec. It's a compromise between product yield and product purity.
I tried to explain the concept of vapor pressure to Juan, but based on his reaction, perhaps I did a poor job. Clearly not seeming to grasp the concept, Juan began to show disinterest and even signs of hostility.
So I decided to take another approach.
"Senor Juan, maybe we can permanently fix the problem by applying some process engineering technology. Shall I explain?"
Juan said no, but I explained anyway.
Two-stage distillation process
"Senor Juan, we are going to create a two-stage distillation process using a partial condenser that will run continuously, process the pisco on-spec all the time, and cost almost nothing to install. We can do it today."
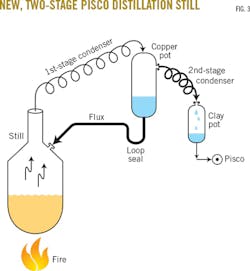
Grasping the concept of an immediate remedy at little cost, Juan smiled and agreed to the project, which I explained would involve four simple steps:
• Cutting the long coil of copper tubing in half.
• Using the first half of copper tubing to connect the still to the vertical copper pot (Fig. 3).
• Leaving the second half of copper tubing connected to the top of the vertical copper pot and having it drain, by gravity, into a clay pot.
• Using a 2-ft long rubber loop seal to continuously drain back the condensed liquid accumulated in the vertical copper pot (reflux) into the still (Fig. 3).
"Senor Juan, the liquid that drains back into the still-called flux, or reflux-will be revaporized in the still. The purpose of the loop is to keep vapors from the still from backing up into the clay pot's liquid drain line, which would stop the liquid draining through the hose. That is, the vapor would push the liquid back into the pot."
"But, Senor Norm, suppose the pisco is still too weak. What should I do then?"
"Juan, just spray some water on the copper tubing between the still and the vertical pot." With the coil acting as a first-stage condenser, I explained that spraying water on it to remove more heat would make more reflux but less product.
"You see, Juan, when I say to cut the long coil of copper tubing in half, I'm just guessing about reducing the size of the existing first-stage condenser (Fig. 2). Maybe it should be a little bit longer to generate the right amount of reflux. But using an external source of cooling, the water spray, will allow you to compensate for lack of cooling by the air."
"But, Senor Norm, what should I do if the pisco becomes too strong?"
"Juan, just add more firewood to the still. That will make more pisco product, too, by distilling over some extra water."
I helped Juan carry out the simple project and, as far as I know, to this day the plant is producing on-spec Pisco using the two-stage distillation column.
Distillation column origins
The technology of the partial condenser to produce a very strong alcoholic beverage originated in Arabia around AD 1000. The patent still-the multistage, multitrayed distillation tower we still use today-originated in 18th-century Scotland. It had a reboiler, condenser, six bubble-cap trays, and a feed preheater. Just a few years ago on a farm in Somerset, England, I saw such a still used to make apple brandy, and it worked just fine.
Only three things have changed in distillation since the 18th century: we now refer to flux as reflux; the still now has a separate reboiler; and we use valve or grid trays instead of bubble-cap trays.
The idea for improving the pisco production technology was not original. I had reproduced the small, two-stage, homemade distillation apparatus I had seen in a storefront window of a liquor shop in Vilnius, Lithuania, a few years before my visit to Peru.
Removing naphtha's tail
Back at the Illinois refinery, after sharing the pisco distillery story, I informed John we could execute the same project at his troubled crude unit.
"First, we'll shut off three of the air cooler's four fans. Then, we'll take the liquid that was partially condensed in the air-cooler outlet header box and drain it back into the flash drum, kind of like reflux. We can route the remaining naphtha vapor to the overhead of the crude tower and comingle it with the crude tower overhead naphtha (Fig. 4). The air cooler will be like the first-stage condenser in Peru, which also will work like a theoretical fractionation stage."
"I don't know if I like that, Norm," John said. "By using the air cooler as a partial condenser, I'm going to wind up with less naphtha from the preflash tower."
"Yes, John, but it's all a balance between product yield and product purity," I said. "It's kind of like being married. You have to trade a part of your freedom for companionship."
We executed the low-cost modifications to the unit just as I'd described them to John, and almost immediately the crude tower overhead was delivering naphtha with an ASTM D-86 distillation endpoint of 380° F. to the reformer, resolving the catalyst-coking issue.
Ongoing applications
The basic process technology used to solve the problems at the Peruvian distillery and the refinery are principles engineers and operators working at a crude distillation unit should apply when considering issues with reflux.
Shortly after the Illinois project, I came upon an operator at the Louisiana refinery I used to manage dealing with a reflux problem.
"Bobby, why are you increasing the reflux on the depropanizer tower?" I asked.
"Morning, Mr. Lieberman. I'm increasing the reflux because there's too much C4 in my propane LPG."
"Okay, that's good, Bobby, but how about the reboiler duty? The steam flow to the reboiler is on manual, so you'll have to increase the steam yourself."
"No, Mr. Norm. According to this morning's lab report, I only have 2% propane in my butane bottoms product, which is right on spec. I don't need more reboiler steam."
"Bobby, we've discussed this before, just last week. You can't just crank up the reflux rate without also increasing the heat to the reboiler. Remember?"
"Uh, actually no, Mr. Norm," Bobby said. "Maybe you can explain that again?"
"Of course I can, Bobby, but let me try to explain it a different way this time, and I'll illustrate the point with some examples. In fact, I'll explain it to you just the way I explained it to John Henry to help him with his crude unit and preflash tower."
I shared the story of John's problem with off-spec naphtha as well as the story of the Peruvian still.
"You certainly are quite the world traveler," he said. "Imagine working all the way down there in Peru!"
"Travelling has its good and not-so-good points. But the point of the stories is that reflux comes from the reboiler. If you increase the tower-top reflux to reduce C4s in your LPG, Bobby, the reflux drum is going to end up empty unless you refill it with vapors generated from the steam reboiler. If the drum reaches empty, the reflux pump is going to lose suction and cavitate, and you'll tear up the pump seal again."
"I'll remember the stories, Mr. Lieberman, as well as always to add steam to the reboiler when I add reflux to the depropanizer," Bobby said. And from then on, he did.
General Introduction
Geared to young and seasoned professionals alike, "Beyond back-to-basics: Process principles and concepts" is a new series of articles designed to present a straightforward approach to mastering the principles and concepts all process engineers should be able to apply without the need of a computer.
While simulations and models are useful for examining long-term operational issues, they cannot replace the dimension of human logic and reason required when tackling the array of complex-and sometimes life-threatening-situations that occur in process plants.
Using experiences from the author's more than 50-year career in the process industry, articles in the series will provide approaches to understanding core process concepts in ways that will equip the engineer to walk out of an office, into a plant, and directly resolve process deficiencies via small operational changes or simple retrofits.
This is the second article in the series.
Distillation safety note
Operating with a high liquid level can result in overpressurizing a distillation tower, which could trigger an emergency opening of a relief valve and subsequent release of liquid hydrocarbons. This may lead to fatal consequences (e.g., the Mar. 23, 2005, fire and explosion at BP PLC's former Texas City, Tex., refinery that killed 15 people and injured 180 others).
To verify the liquid level, place a pressure gauge on the top of the gauge glass. Next, note the tower-top pressure on the panel. To obtain the height of liquid (ft) above the top of the gauge glass, multiply the difference (psi) by 2.3 (ft) and divide by specific gravity.
This calculation is necessary because level indications on both the panel and gauge glass are not reliable, especially if specific gravity is variable due to temperature or composition.