P. 4 ~ Continued - Mixing MDEA, TEA shows benefit for gas-sweetening operations
View Article as Single page
Conversely, CO2 reacts more slowly than H2S and forms stable reaction products that do not depend on alkalinity, as it is the case for H2S ones. Kinetically, if both amine and acid gas were exposed to each other for a short residence time, H2S will react more rapidly than CO2 due to its instantaneous reaction that keeps the unreacted form of H2S low in the liquid phase, which helps to maintain high driving force. A different scenario, however, occurs if the CO2 reaction rate was high enough to prevent H2S concentration buildup. Higher absorption rate of CO2 will consume the amine and reduce its alkalinity.
This trend is maintained until the solvent alkalinity is too low to keep all H2S in a protonated form. Consequently, the solvent favors CO2 products that are driven through an almost irreversible reaction and allows the de-protonation of HS.– and desorption of H2S.19 As discussed earlier, primary and secondary amines have higher CO2 rates of reaction constants than TEA, hence better prevention of H2S concentration buildup.
These facts are well depicted by both Fig. 2a, where such mixtures as DEA-MDEA and DIPA-MDEA produced sweet gas with higher H2S contents than that produced by the tertiary-amine mixture MDEA-TEA. This analysis can also be used to explain the dramatic increase in the reboiler duty for MDEA-MEA mixture. MEA rate of reaction constant is about 1,200 times that of MDEA, which testifies to its high ability fully to absorb CO2 at a given short residence time. Literature review done by Ma'mun shows that CO2 partial pressure with 30 wt % MEA at a temperature range of 25-120o C. is measured to be in the range of 0.2-6616 kPa compared with 0.00161-6570 kPa for a solution of MDEA 50 wt % at the same temperature.20
Comparison between pure MDEA solvent and MDEA-TEA mixture can be made based on the acid-gas loading capacity. Table 1 shows that MDEA and TEA kinetics are close due to their similar chemistry. Table 4, however, demonstrates that MDEA has a higher acid-gas loading capacity than TEA. This explains better why MDEA-TEA mixture absorbs less H2S and CO2 than pure MDEA solvent.
Different paths are available to take advantage of the reduced reboiler duty achieved by the mixture MDEA-TEA while keeping the sweet-gas specifications similar to those produced by single 45 wt % MDEA solvent. These paths include increasing the amine circulation rate or decreasing the trim-cooler temperature of 61o C. In this study, we chose optimization of the trim-cooler temperature because increasing the amine circulation rate is believed to be less cost effective due to the large difference between both solvents' loading capacities.
Goharrokhi shows data calculated with Chakma's correlation, in which it is obvious how the equilibrium constant in the MDEA protonation reaction gets higher with lower reaction temperature.21 In other words, decreasing the trim-cooler temperature shifts the equilibrium of the exothermic reaction forward, thus increasing the absorption rate. The absorption rate constant can be expressed mathematically with Equations 7 and 8 as functions of temperature for MDEA and TEA, respectively.10
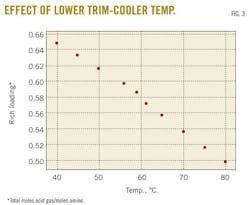
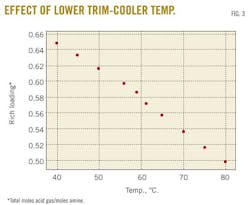
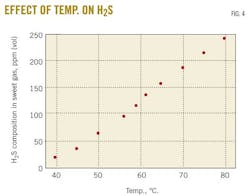
A case study predicted changes in the rich-amine loading by varying the trim-cooler temperature in the range 40 to 80o C. Fig. 3 demonstrates that decreasing the cooler temperature leads to an increase in rich-amine loading, thus a decrease in H2S and CO2 concentrations in sweet gas. Fig. 4 shows the change in H2S concentration in the sweet gas as function of temperature.
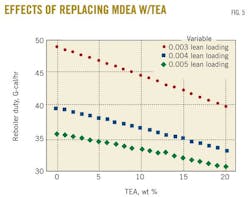
Finally, another case study verified the same conclusion can be drawn for different lean-amine loadings through quantifying their corresponding reboiler duties. Obviously, a lower lean loading requires a greater energy to regenerate the amine. This offers a stronger driving force through which absorption takes place and a sweeter gas product is obtained.
Fig. 5 shows that the same trend was obtained with different lean-amine loadings with almost equal slopes for lean-amine loading of 0.005 and 0.004. As the lean-amine loading decreases, a higher reduction in the reboiler duty is achieved. Indeed, for 20% of MDEA replaced by TEA, the reboiler duty reduction is calculated to be 16% and 18.5% when lean-amine loading passes from 0.004 to 0.003.
Displaying 4/6
View Article as Single page