In oilfield chemistry, green is the color of progress
Bevin Parks-Lee and Steve Fidoe
Champion Technologies, Fresno, Calif.
A green revolution is gaining ground in the oilfield chemicals industry, where core corporate values have expanded to encompass environmental stewardship and sustainability.
Leading oilfield chemical companies are redefining the meaning of green by drawing upon green chemistry principles and practices first articulated in the 1990s to implement more environmentally-sustainable policies and procedures in laboratories, manufacturing facilities, warehouses, and logistical operations.
The definition of green is in the eye of the beholder. Chemists, manufacturers, regulators, and oil and gas producers all advocate green advances, but each has a distinct emphasis. While a chemist might focus on improving details of a reaction in order to improve yield or decrease waste, a manufacturer might be more focused on energy consumption or availability of starting materials. Similarly, a regulator might be more focused on the health and welfare of people and the environment, while a producer might be more concerned about performance of production chemicals in a new, more challenging environment. The common purpose linking green intentions, whoever or whatever drives them, is sustainability.
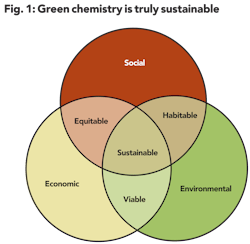
Sustainability is simply meeting today's needs without compromising the ability of future generations to meet their needs. This can be visualized as three overlapping areas of interest: social, environmental, and economic (see Figure 1). In this concept, if a new chemical technology is both socially and environmentally acceptable, an ecosystem will remain habitable. If the technology is environmentally acceptable and economically feasible, then it is viable.
Where the economic and social impacts balance, there is an equitable marketplace. Sustainability is achieved when a viable technology can contribute to market equity while preserving the environment. All three conditions are necessary for sustainability to result.
Green chemistry, the process
In the world of oilfield chemicals, green principles and practices can be applied at literally each step of the manufacturing process, from development through deployment.
From a process perspective, a practice as fundamental as using renewable feedstocks qualifies as green chemistry, just as much as designing chemical products that degrade into innocuous substances after use or treatment strategies that minimize the volume of chemical needed to achieve performance objectives. Using raw materials and feedstocks that are renewable—such as agricultural or industrial wastes—is more sustainable than using depletable materials, such as fossil fuels or mineral resources.
Laboratory processes offer almost limitless opportunities to implement green chemical concepts. Chemists can design chemical syntheses that use and generate substances with little or no toxicity to living things or their habitats, or they can design syntheses with more efficient synthetic pathways or atmospheric reaction conditions. The safety benefits of creating and working with less toxic substances in the lab are direct, while leaving little or no waste to treat or clean up saves time, money, and environmental resources and therefore is more sustainable.
An effective way of minimizing waste, in the laboratory or manufacturing plant, is to optimize the amount of material going into a reaction with respect to what comes out as product. Catalysts, which by definition are used in small amounts, can carry out a single reaction multiple times. These are preferred over stoichiometric reagents, which must be used in equivalent if not excess amounts and work only once. Blocking or protecting groups or temporary modifications during synthesis should be avoided because such derivatives require the use of additional reagents and generate more waste.
Green chemistry principles and practices being implemented at laboratories around the world protect the bottom line, safeguard employees, and preserve the environment:
- Using safer solvents and separation agents and safer reaction conditions.
- Running chemical reactions at ambient temperature and pressure whenever possible to improve energy efficiency.
- Analyzing reactions in real time during syntheses to minimize or eliminate the formation of byproducts.
- Designing reactions to minimize the potential for chemical accidents and releases to the environment.
- Minimizing waste by optimizing product yields.
Focus on performance
Understandably, most participants contributing to the process of introducing more environmentally acceptable chemistries to upstream oil and gas operations are focused on performance at the point of delivery.
The most visible benefit of the green chemistry principles and practices some oilfield chemical companies are implementing has been the introduction during the past decade of whole families of more environmentally acceptable specialty chemicals. Green oilfield chemicals today are much more effective than many oil and gas producers believe possible.
Many new green chemistries are proving to be effective at lower dosage rates, a performance characteristic that further reduces their environmental impact and helps hold down costs. This is especially important because, to be sustainable, green chemical treatment costs must be competitive with the cost of treatments using the chemistry being replaced.
Not only are the benefits of improved performance easily documented, better performance of green chemicals may also translate into better protection of the environment, safer work places, and economic viability. However, the focus on performance introduces a lot of complexity to the goal of developing new environmentally acceptable chemicals, because:
- Environmental-protection rules vary considerably, from one state, province, or country to the next, creating a labyrinth of dissimilar technical requirements and regulatory objectives.
- Extreme variability of logistical requirements introduces unique handling, delivery and deployment consideration and costs at each production site.
- Each operator has different production objectives and unique economics. A solution that is effective for one operator might not work for another.
Regulatory systems endeavor to protect the environment through unique sets of rules and reporting requirements, and each system has varying technical standards. For example, the primary objective of a regulator under one set of rules might be to ensure that only chemistries with specific intrinsic properties are introduced at the well site; while another regulatory regime might be most concerned with the impact of substances – both process chemicals and trace levels of hydrocarbons discharged into the environment. Similarly, regulatory regimes in some cases place ultimate responsibility with the operator for ensuring that only chemicals meeting specific requirements are applied at the production site or that no banned by-products are disposed of into the environment. In both instances, regulators and producers—even environmental advocates—tend to consider only chemistries that satisfy environmental-protection requirements to be green.
To be sure, the performance of green chemicals is important to oilfield chemists. But green chemistry principles teach that the environmental, safety, and economic benefits of green chemistry begin to accrue far from the application site. This holistic framework stresses sustainability, efficiency, and safety in the workplace and expands the definition of green chemistry to encompass much more than better environmental stewardship at the well site.
The many meanings of 'green'
Parallel to the adoption of more efficient, more sustainable chemical practices and processes, the green chemistry movement has provided the impetus for some oilfield chemical companies to comprehensively upgrade the equipment and instruments in their labs. In the process, many proven but outdated experimental methods have been discarded for more rigorous, more accurate procedures based on green chemistry concepts. This new infrastructure of green principles, practices, and state-of-the-art facilities is developing and deploying more environmentally acceptable chemicals to solve an ever wider range of both familiar and exotic production challenges.
Importantly, oilfield chemical companies that have adopted green chemistry principles and implemented green practices in their labs begin to accrue benefits very early in the process, as the value created by more sustainable operations begins impacting the bottom line. Competitive economics are essential to sustainability for both operators and oilfield chemical companies.
Green chemistry has invented and continues to define a new context for more detailed study of oilfield chemicals. Better understanding of the safety and environmental hazards oilfield chemicals can introduce to the well site is being matched with the development of new green chemistries to deal with the dangers. Ultimately, green chemistry is a means of taking responsibility for protecting the environment in the most comprehensive way possible, by preventing damage before it occurs.
In this new context, green is more than just the color an oilfield chemical's environmental attributes. Green is also the color of the processes and practices that enable development of new chemistry, and green is the color of the economic imperative any technology must achieve to be sustainable.
Today more than ever before, green is the color of sustainable oilfield chemistry.
The authors wish to thank Cassandra Smith in Champion's UK regional headquarters Aberdeen, Scotland, for her contributions to this article.
About the authors
The 12 principles of green chemistry
The term "green chemistry" was first used in the early 1990s by Paul Anastas, formerly of the EPA and the White House Office of Science and Technology Policy. Anastas, who helped create the Green Chemistry Institute in 1997, assumed the post of director, Center for Green Chemistry and Green Engineering, at Yale University in 2007.
John C. Warner of the University of Massachusetts department of chemistry and Anastas co-authored the book Green Chemistry: Theory and Practice (Oxford University Press: New York, 1998), in which they enunciated the following 12 principles of green chemistry, which stress sustainability, efficiency, and environmental safety:
1. Prevent waste: Design chemical syntheses to prevent waste, leaving no waste to treat or clean up.
2. Design safer chemicals and products: Design chemical products to be fully effective, yet have little or no toxicity.
3. Design less hazardous chemical syntheses: Design syntheses to use and generate substances with little or no toxicity to humans and the environment.
4. Use renewable feedstocks: Use raw materials and feedstocks that are renewable rather than depleting. Renewable feedstocks are often made from agricultural products or are the wastes of other processes; depleting feedstocks are made from fossil fuels (petroleum, natural gas, or coal) or are mined.
5. Use catalysts, not stoichiometric reagents: Minimize waste by using catalytic reactions. Catalysts are used in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and work only once.
6. Avoid chemical derivatives: Avoid using blocking or protecting groups or any temporary modifications if possible. Derivatives use additional reagents and generate waste.
7. Maximize atom economy: Design syntheses so that the final product contains the maximum proportion of the starting materials. There should be few, if any, wasted atoms.
8. Use safer solvents and reaction conditions: Avoid using solvents, separation agents, or other auxiliary chemicals. If these chemicals are necessary, use innocuous chemicals.
9. Increase energy efficiency: Run chemical reactions at ambient temperature and pressure whenever possible.
10. Design chemicals and products to degrade after use: Design chemical products to break down to innocuous substances after use so that they do not accumulate in the environment.
11. Analyze in real time to prevent pollution: Include in-process real-time monitoring and control during syntheses to minimize or eliminate the formation of byproducts.
12. Minimize the potential for accidents: Design chemicals and their forms (solid, liquid, or gas) to minimize the potential for chemical accidents including explosions, fires, and releases to the environment.
More Oil & Gas Finacial Journal Current Issue Articles
More Oil & Gas Financial Journal Archives Issue Articles