Challenges of methane hydrates
Abundant but difficult to exploit, are gas hydrates the next major energy source?
Darren Spalding and Laura Fox, Bracewell & Giuliani LLP, London
The energy of the future could lie buried deep beneath the world's oceans and the Arctic permafrost. Methane hydrates, also known as "flammable ice," are vast reservoirs of natural gas trapped in ice-like crystals and hold the potential to alter trade flows and reshape the geopolitics of energy.
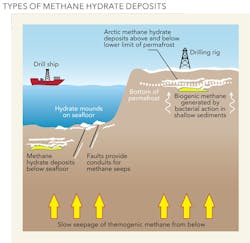
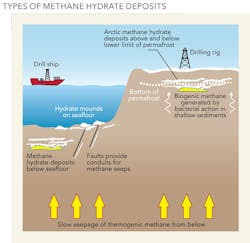
As the name suggests, methane hydrates consist of a methane molecule surrounded by a cage of interlocking water molecules. Hydrates store large amounts of gas in a relatively small area; one cubic meter of hydrate can hold around 160 cubic meters of methane and 0.8 cubic meters of water. Methane hydrates are similar to ice in their composition and occur naturally in subsurface deposits in freezing temperature and high pressure conditions.
The sea floor is thus an ideal location for their formation: the deep seabed is uniformly cold, with temperatures from zero to four degrees Celsius, and below a water depth of about 350 meters, the pressure is sufficient to stabilize the hydrates. When melted or exposed to pressures and temperatures outside those where the ice is stable, the solid crystalline lattice turns into liquid water, and the enclosed methane molecules are released as gas.
Until recently, methane hydrates had never been tapped as a source of energy to meet increasing global demands. It has generally been considered that other sources of fossil fuels, notably conventional oil and gas (and more recently shale oil and gas), have been easier and cheaper to access. But in March 2013, Japan became the first country to successfully flow gas from methane hydrate deposits under the Pacific Ocean.
Japan kick-starts exploration
JOGMEC, the Japanese energy organization, estimates that there is some 1.1 trillion cubic meters of methane held in methane hydrate deposits in marine sediments in the Nankai Trough off the Pacific coast of central Japan. This is equivalent to around 11 years of the amount of LNG that is currently imported into Japan. A further 120,000 cubic meters of gas from methane hydrates has been discovered some 50 miles off the coast of Aichi Prefecture in central Japan following recent tests. These resources could represent just the tip of the iceberg.
Such discoveries are even more significant for Japan once the repercussions of the 2011 Fukushima Daiichi disaster are taken into account. Indeed, all but two of Japan's 54 nuclear power plants (which once provided 30% of the nation's energy) have ceased operations. With virtually no exploitable domestic fuel sources to take the place of nuclear, Japan is therefore increasingly reliant on expensive imports of oil and LNG. In 2011 Japan consumed 123 billion cubic meters of natural gas, of which 117 billion cubic meters were imported.
The implications of methane hydrates as a new energy source for countries lacking access to conventional resources are therefore profound. It is thought that methane hydrates within Japan's territorial waters may be able to supply the nation's natural gas needs for a century. For Japan, historically resource-poor, domestically produced methane hydrate gas has the potential to reduce the dependence on imports that has defined its energy system throughout the modern era.
Potential environmental impacts
While methane hydrate resources may appear to be an enormous boon to energy-hungry nations like Japan, it is widely considered that developing methane hydrates could have a significantly detrimental effect on the climate if it results in the escape of methane into the atmosphere. Additionally, the role that methane hydrates play in stabilizing the seafloor should not be underestimated. For example, drilling deep into oceanic deposits could impact both marine life and the seabed, potentially causing sediment to slide down the continental slope. Some evidence suggests that such underwater landslides have already occurred. Geologists consider that the movement of so much sediment could also trigger tsunamis.
Further, methane is a greenhouse gas and is considered to be 25 times more potent than carbon dioxide in trapping solar radiation in the atmosphere. Scientific studies have revealed that the gas has already started to leak from oceans and soils in the Arctic. As a result, there is concern over any uncontrolled release of methane from hydrate formations. Methane hydrates are particularly fragile: the sediment in which they are located is inherently unstable once removed from the high pressures and low temperatures of the deep sea. Any dissociation can result in leakage and extraction is, at present, inefficient.
However, cleaner-burning gas from hydrates may help to displace coal consumption in countries such as China and India, just as cheap shale gas is now driving some coal out of the American electricity markets. This scenario could potentially yield climate benefits and cleaner air – assuming the displaced coal stays in the ground. Nevertheless, even modest leakage rates could nix any potential climate benefit of burning hydrates instead of coal.
Potential ecological and environmental risks are being flagged. The considerable rewards of releasing methane from gas hydrate fields must therefore be balanced with risks, and more research may be required to determine the likelihood of such risks materialising and whether there are ways of mitigating them. Some researchers say that they ultimately expect the issues to be comparable to those of offshore conventional natural gas production.
Technology plays its part
The high cost of LNG imports has given Japan greater motivation to press ahead with its methane hydrate research program, the value of which is currently in the region of US$700 million. Researchers in Japan hope to develop production technology that achieves controlled release of the methane from the ice into the production well, thereby minimizing the risk of gas escaping into the atmosphere. But despite Japan's ground-breaking success in producing gas from undersea hydrates, there is still much work to be done to develop a commercially profitable set of technologies for efficient methane hydrate extraction.
Yielding commercial quantities of natural gas from hydrates at an affordable price presents numerous challenges. Such challenges stem in part from the difficult environments in which hydrates are found and where would-be hydrate producers must drill: the frozen expanses of the Arctic and the deep sea abyss.
A variety of technologies could be developed to produce methane hydrates using pressure reduction, ion exchange and other processes that take advantage of their unique chemical and physical properties. The US, Canada, Japan, and India all have vigorous research programs working to discover viable technologies. Most of these research programs remain in the exploratory, experimental, and laboratory phases.
Despite recent advances, commercial production is still unlikely for at least 10 to 15 years. Japan believes that commercial production will be possible by 2018, while the US Geological Survey estimates that countries with the "political will" to pursue methane hydrates could see production by around 2025.
The cost of developing any new energy is high and methane hydrates are no different. The current cost of gas produced from methane hydrates is estimated to be US$30 to US$50 per million British thermal units (MMBTUs), compared to the current Henry Hub price of approximately US$6 per MMBTU. The International Energy Agency estimates that once efficient practices and processes are developed, natural gas produced from methane hydrates will cost between US$4.70 and US$8.60 per MMBTU.
The future
The world's demand for energy continues to increase rapidly and global energy consumption is expected to rise by 41% between 2012 and 2035. Among fossil fuels, gas demand is growing fastest and is increasingly being used as a cleaner alternative to coal for power generation as well as in other sectors. Any potential new source of gas production is therefore likely to be given considerable attention by both industry and government.
Methane hydrates may represent the world's largest source of untapped fossil energy. They are believed to be a larger hydrocarbon resource than all of the world's oil, natural gas, and coal resources combined. Of course, just as with shale gas (and conventional hydrocarbons, for that matter), not all of this potential energy resource will prove technically recoverable. Yet if the technology to commercially extract gas from hydrates is developed, the implications for global energy markets are staggering.
About the authors
Darren Spalding is a partner in Bracewell & Giuliani's London office. He advises clients on upstream, midstream, and downstream oil and gas M&A transactions, with an emphasis on cross-border deals, having advised on transactions involving assets in more than 30 jurisdictions.
Laura Fox is an associate in the firm's London office. She is a transactional lawyer with experience handling syndicated and bilateral lending transactions, secured lending transactions, restructurings, as well as trade finance and project finance transactions.