TECHNOLOGY New line pipe resists preferential corrosion at welds
Sigeru Endo, Sakae Fujita
NKK Corp.
TokyoMoriyasu Nagae
NKK America
New YorkOsamu Hirano
NKK Corp.
Fukuyama
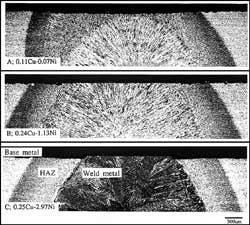
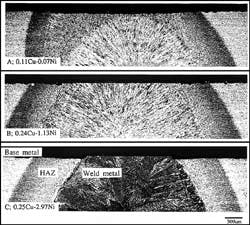
Microstructures of immersion-tested specimens (Fig. 1).
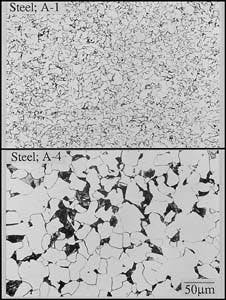
Microstructures of base metal of A-1, A-4 specimens (Fig. 3).
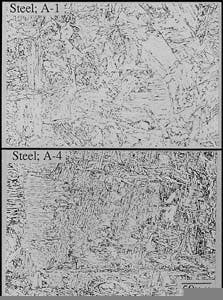
Microstructures of HAZ of A-1, A-4 specimens (Fig. 4).
Cross section of a girth-welded joint of an immersion-tested specimen (GMAW; Fig. 9).Line pipe with preferential corrosion resistance in the welded joint in CO 2-containing gas and oil environments has been produced and delivered for use in the North Sea.
NKK Corp., Tokyo, developed the pipe by taking into consideration the mechanism of preferential corrosion. The chemistry was optimized to prevent preferential corrosion of both heat-affected zone (HAZ) and weld metal.
The project also developed girth-welding consumables for the pipe.
Increasing CO2
CO2 corrosion problems of line pipe and oil country tubular goods (OCTG) have been increasing with the expanding number of oil and gas wells containing CO2.1-3 Corrosion rates in a CO2 environment are much higher than in other corrosive environments having similar pH values because of the buffering effect of the solution.4
Weld metal and HAZ areas are much smaller than the base metal surface area. Therefore, accelerated corrosion is anticipated when preferential corrosion of the weld metal or HAZ occurs.
In fact, severe weld-metal corrosion was detected in a North Sea submarine pipeline transporting oil containing a small amount of water and CO2.5 6
Immersion tests, polarization measurements, and a potential-distribution analysis of the inner pipe surface clarified the mechanism and morphology of the preferential corrosion.
These results show that a possible mechanism is galvanic corrosion caused by differences in the immersion potentials of the weld metal, HAZ, and base metal.
Moreover, the weld-metal chemistry as well as the base-metal chemistry also affect the corrosion morphology.7 8
Table 1 [8315 bytes] shows the targeted properties of the preferential corrosion-resistant pipe. Both the strength of the API X-65 Grade and the toughness of ordinary offshore line pipes were used as the targeted properties of the pipe.
A maximum hardness in the weld joint was also set to reduce susceptibility to sulfide stress-corrosion cracking (Table 1). The maximum hardness in the T-cross area was also set at the same value as that of the submerged arc weld (SAW) longitudinal seam.
Corrosion: welded joints
Fig. 1 shows macrostructures of welded joints after immersion in sea water saturated with 0.1 Mpa CO2 for 2 months. The Ni content of the weld metals varied.
Weld Metal A, which contains less Ni than the others, shows a higher corrosion rate than the base metal. The difference in the corrosion rate between the weld metal and the base metal is approximately 0.5 mm/year.
On the other hand, Weld Metals B and C have lower corrosion rates than the HAZ and base metals.
The preferential corrosion mechanism is galvanic corrosion caused by the difference in immersion potentials of the base metal and weld metal. This difference arises from differences in their chemical compositions.
When preferential corrosion occurs, a galvanic current that is expressed by Equation 1 flows between the macroanode and macrocathode.9
Ig = Iaa - Iac = Icc - Ica (1)
where: Iaa, Iac = Anodic and cathodic currents at the macroanode, respectively; Ica, Icc = Anodic and cathodic currents at the macrocathode, respectively.
Measurement of the galvanic current 1g allows the location of the macroanode and macrocathode to be determined in a welded joint.
Furthermore, the portion having higher electrochemical potential cannot be preferentially corroded because galvanic corrosion arises from the difference in the immersion potentials.
Table 2 [26568 bytes] shows the compositions of the base metal and weld metal of the test specimen. The galvanic currents and the immersion potentials of the base metal, HAZ, and weld metal were measured in 30° C. synthetic sea water saturated with 0.1 Mpa CO2.
Fig. 2 [23243 bytes] shows the measured values. A base-metal specimen and a weld-metal specimen were separated from a welded joint, then mounted in an epoxy resin. A simulated HAZ specimen was also mounted in the epoxy resin with the base metal and the weld metal specimens.
The specimens were connected by copper wire to measure the galvanic current. The galvanic currents between the base metal and both the weld metal and HAZ were measured, and the galvanic current densities of each region calculated.
In this study, the signs of the weld metal and of the HAZ galvanic current are positive when preferential corrosion of the weld metal and HAZ does not occur.
Weld metal with 1.1% Ni and 0.2% Mo shows a higher immersion potential than base metal and HAZ; thus, the sign of the weld metal galvanic current is positive.
Corrosion: HAZ
Polarization measurements were carried out on the six steels with chemistries listed in Table 3 [24755 bytes] to examine the effect of base-metal chemistry and HAZ microstructure on the HAZ preferential corrosion characteristics.
Additions of Cu, Ni, and sulfide-shape-control elements such as Ca are reported to be effective in preventing the grooving corrosion of weldments of electric-resistant-welded (ERW) pipes. Reduced S is also effective to prevent this corrosion.10-13
Therefore, the C, S, Cu, Ni, and Ca contents of the base metals were varied as shown in the table to verify the effects of these elements.
Base metals with two types of microstructures were evaluated. The microstructures of A-1, A-2, and A-3 were bainite; those of A-4, A-5, and A-6 were ferrite-pearlite.
Both base metal and HAZ polarization properties were measured in the CO2 environment described previously. Simulated HAZ specimens were made with a thermo-mechanical simulator and used for the polarization measurements.
In the HAZ simulation, a thermal cycle that consisted of heating to 1,350° C. and cooling at 20° C./sec between 800° C. and 500° C. was applied to the six steels.
Fig. 3 demonstrates the microstructures of the A-1 and A-4 base metals. Fig. 4 shows typical HAZ microstructures for A-1 and A-4.
Although small differences in the HAZ microstructures were apparent, the microstructure was mainly bainite, regardless of base-metal chemistry. The HAZ microstructures for A-2, A-3, A-5, and A-6 are also bainite.
Fig. 5 [92736 bytes] shows polarization curves for A-2 and A-3. Both the base metal and HAZ polarization curves are illustrated in these figures.
No significant differences were observed in the immersion potentials of the base metal and the HAZ of Steel A-1. By contrast, the immersion potentials of the HAZ of Cu and Ni-containing Steels A-2 and A-3 were higher than those of the base metals.
Furthermore, the largest difference between the immersion potentials of the HAZ and the base metal appeared in A-3, which had lower S and contained Cu and Ni.
Therefore, the addition of Cu and Ni and the reduction of S were effective for increasing the immersion potential of the HAZ over that of the base metal. The HAZ potentials in Steels A-4, A-5, and A-6 had lower electrochemical potentials than those of the base metal.
In addition, significant differences in the polarization curves of both the HAZ and base metal were not observed in those three steels.
These results demonstrate that the immersion potential of the HAZ is higher than that of the base metal in the lower S, Cu, and Ni-containing steels with a bainitic microstructure. Hence, preferential corrosion of the HAZ is unlikely in a CO2-containing environment.
Corrosion: weld metals
Fig. 6a [93764 bytes] shows the effect of Cu and Ni content on the galvanic current density of weld metal.
The difference in Cu and Ni content between the weld and base metals is indicated by the horizontal axis of Fig. 6a. The galvanic current densities of weld metals for SAW, shield metal-arc welding (SMAW), and gas metal-arc welding (GMAW) welded joints are shown here.
Fig. 6a shows that, regardless of the welding method, the difference in both Cu and Ni content has a strong correlation to the galvanic current density and that the additions of Cu and Ni are effective for increasing the galvanic current density.
The galvanic current density is positive when the difference in Cu and Ni is more than 0.5%. This shows that the addition of Cu and Ni is effective in preventing preferential corrosion of weld metals in a CO2-containing environment, regardless of the welding method.
Fig. 6b shows the corrosion rates for both base metal and weld metal of a GMAW welded joint in synthetic sea water saturated with 0.1 Mpa CO2.
The corrosion rate of the weld metal decreases with an increase in the difference in Cu and Ni content of the weld and base metals.
No significant difference in the corrosion rate of the base metals due to the difference in Cu and Ni content was apparent. The corrosion rate of the weld metal became less than that of the base metal; therefore, no preferential corrosion of the weld metal was apparent for weld metals with a Cu and Ni content of at least 0.5% more than that of the base metal.
The preferential-corrosion tendency observed in the immersion test shows good correlation with the electrochemical test results. The difference in Cu and Ni content required to prevent preferential corrosion of weld metals was 0.5% in both the electrochemical test and the immersion test.
Fig. 7 [19992 bytes] shows the effect of varying the Mo content on the galvanic current density. Three welding methods (SAW, SMAW, and GMAW) were used to make welded joints. As Fig. 7 shows, small Mo additions, such as 0.05%, appear to be sufficient to prevent preferential corrosion of the weld metal.
Resistant pipe, consumables
A Cu and Ni-containing bainitic steel was selected as the base metal based on the test results previously mentioned, and optimization of the longitudinal-seam weld metal chemistry was carried out.
The mechanical properties of the weld metal were the primary concern.
Fig. 8a [98584 bytes] shows the relationship between the PCM (center of the weld metal) of the weld metal and the maximum hardness of the T-cross test.
Maximum hardness increases with increasing PCM value. No significant effects of the addition of Ni or Mo on the hardness were observed.
The weld metal, with a PCM of 0.18, shows the highest absorbed energy in the Charpy test. The results indicate that the PCM of the longitudinal seam weld metal should be controlled for both low temperature toughness and low hardness of the T-cross portion (for example, to meet an Hv 260 maximum requirement).
Thus, Mo additions to the longitudinal seam weld metal appear to satisfy both mechanical properties and preferential-corrosion-resistance properties of the weld metal. The Mo content required to prevent preferential corrosion is much less than that of Ni and Cu; hence, the increment in the PCM value by Mo additions is smaller.
This indicates that both excellent preferential corrosion resistance and mechanical properties can be obtained by using the Mo-containing weld metal.
Fig. 8b shows the toughness of SMAW weld metals as an example of the mechanical properties. Both the maximum hardness and toughness of the weld metals correlate well with the PCM value of the weld metal. The hardness increases and the toughness deteriorates with increases in the PCM value.
The mechanical property results clearly show that control of the PCM value of the weld metal is important for obtaining adequate mechanical properties. SMAW weld metals with a PCM less than 0.24% and GMAW weld metals with a PCM less than 0.18% satisfy the targeted mechanical properties.
Mechanical, corrosion properties
Based on the test results described, trial production of the preferential corrosion-resistant UOE pipe, along with its longitudinal seam and girth weld consumables, was carried out.
Table 4 [26963 bytes] shows the chemical composition of the base metal and longitudinal weld metal of the developed preferential corrosion-resistant UOE pipe.
Table 4 also shows chemical compositions of the girth-weld metals made with the developed SMAW and GMAW welding consumables. The accelerated cooling process was employed to obtain the bainitic microstructure of the base metal.
Table 5 [24755 bytes] shows the mechanical properties and immersion test results of the welded joints and weld metals. The mechanical properties satisfy the targeted values mentioned previously.
As shown in Fig. 9, no preferential corrosion was observed in the immersion test.
Conclusions
Based on these test results, 6,000 tons of the preferential-corrosion-resistant pipes were mass produced for a North Sea marine pipeline.
Overall, the testing program led to the following conclusions:
- The immersed potential of the HAZ was clearly higher than that of the base metal in the lower S, Cu, and Ni-containing steels with a bainitic microstructure. Hence, preferential corrosion of the HAZ of this type of steel is unlikely in a CO2 environment.
- The sign of the galvanic current density is positive when the chemical composition difference between the weld and base metals in Cu and Ni are greater than 0.5% and that of Mo is above 0.05%.
- This indicates that the addition of Cu and Ni or of Mo is effective for preventing preferential corrosion of weld metal in a CO2 environment, regardless of the welding method.
- The mechanical property testing results make clear that control of the PCM value of the weld metal is important for obtaining adequate mechanical properties.
- The mechanical properties of the developed UOE pipe satisfy the aim values. No preferential corrosion of welded joints was observed in immersion testing.
Acknowledgment
The authors thank Takashi Wada of Kobe Steel Co. for his helpful discussions and developing welding consumables.
References
1. Ikeda, A., et al., Corrosion 83, Paper No. 45, 1983.
2. Ikeda, A., et al., Corrosion 84, Paper No. 289, 1984.
3. Videm, K., et al., Corrosion 87, Paper No. 42, 1987.
4. Kodama 47th Fusyokuy-Bosyoku Symposium, 1983, p. 1ff.
5. Steel, S.J.M., et al., OGJ (May 6, 1991), p. 49.
6. London, C.J., Pipes & Pipelines International, May-June, 1991, pp. 7-13.
7. Endo, S., et al., Conference of the Japan Welding Society Annual Meeting, Vol. 50, pp. 246-47 (1992).
8. Endo, S., et al., Quarterly Journal of the Japan Welding Society, Vol. 12, No. 4, pp. 515-20 (1994).
9. Tsujino, B., and Miyase, S. Corrosion, Vol. 37 (1981), p. 540 ff.
10. Miyuki, Zairyo, Vol. 38, No. 424.
11. Katoh, et al., Bosyoku-gijutu, Vol. 23 (1974), pp. 385-92.
12. Katoh, et al., Bosyoku-gijutu, Vol. 23 (1974), pp. 425-32.
13. Itoh, et al., IIW Doc. IX-1421-86.
The Authors
Shigeru Endo is a senior research engineer of the materials and processing research center of NKK Corp. Japan, Tokyo, which he joined in 1985.
Sakae Fujita is a senior research engineer of the materials and processing research center of NKK Corp. which he joined in 1980.
Moriyasu Nagae is a senior engineer of NKK America Inc. He joined NKK Corp. in 1978 in Japan and moved to NKK America in 1996.
Osamu Hirano is a general manager of welded pipe mill at NKK Fukuyama; he joined NKK in 1970.
Copyright 1997 Oil & Gas Journal. All Rights Reserved.