Tests confirm effectiveness of new inhibitor for pipeline internal corrosion
Comparative tests of commercially available corrosion inhibitors revealed that a new corrosion inhibitor, IMP-ICCA-9710, presented a higher protection efficiency in corrosion protection.
Inhibitors were evaluated in rust, electrical resistance probe, bottle, and dynamic circuit (loop) tests; in the latter one, electrochemical and weight loss techniques were used, observing a good correlation in both cases.
The IMP-ICCA-9710 inhibitor turned out to be excellent for pipeline protection against corrosive agents in crude oil, displaying a protective film that adheres to the metal surface for long periods.
The inhibitor is an imidazoline type and dispers able in water and soluble in hydrocarbons, thus permitting protection of pipes in stratified flows.
Availability of the inhibitor comes during a time of increased processing of heavier crudes that present a higher content of salts, sulfur, and clays, for example.
Water contact requires high-efficiency corrosion inhibitors that can achieve less than 2 mils/year (mpy) corrosion rate in stratified or annular flow.
Causes of internal corrosion
Line pipe is designed against internal corrosion, but frequent changes in operating conditions or changes in the type of crude oil or in the volumes being transported, for example, may increase the corrosion rate, leading to increased failures over time.
Following are the main causes of internal corrosion in crude oil pipelines:
- Multiphase flows (crude oil, gas, and water).
- Crude oils containing different salts, water, and sediments in quantities greater than market-established limits (i.e., salt content higher than 30 lb/1,000 bbl). Additionally other corrosive agents such as H2S, mercaptans, and naphthenic acid are present.
- Presence of CO2 and residual oxygen.
- Sulfate-reducing bacteria (SRBs).
- Erosion caused by the presence of solids.
Water is the corrosive agent that causes the major damage to the pipes, with the corrosion rate normally increasing as the water content increases. Other important factors influencing pipe corrosion is metallurgy, as well as pipe operation, such as flow speed, local turbulence, temperature, and pressure, etc. The construction material normally found in (pipe) carbon steel corrodes with these agents.
An important factor causing corrosion is flow speed. Stratified flow causes the main problem because stratified or static water attacks the pipe walls. In this kind of flow, it is difficult for the inhibitor to reach those places where metal is covered by water. In this case, a water-soluble inhibitor results in higher efficiency.
Programs for corrosion control generally combine the following:
- Chemical treatment (inhibitors, biocides, asphaltene dispersing agents, sulfur, and oxygen scavengers, among others).
- Chemical and mechanical cleaning.
- Materials selection.
- Internal coatings (polymers or cements).
- Systems design optimization (speed, geometry, etc.)
- Process conditions control (pressure, pH, temperature, water, etc.).
Crude oil pretreatments in advance of pipeline transportation can decrease corrosion. Corrosion inhibitors, however, have been used for a long time in transporting crude oils. These inhibitors can be divided into two kinds: soluble in oil and soluble in water.
The former are typically used in transporting crude oil and oil and gas production. They are generally carbox ylic acid salts and organic amine.
Water-soluble inhibitors are used in production wells with high water content and in transporting lines. Typical water-soluble inhibitors consist of ammonium quaternaries.
Both types of inhibitors function by forming different types of protective films on the metal surface, perfectly homogeneous and adhered to the metal for an extended time.
This work refers to the development of a new product, which specifically protects pipelines transporting crude oils. Inhibitors were synthesized into different functional groups. The synthesized inhibitor forming the most resistant film and a higher efficiency for protection against corrosive agents was selected.
Experiments
The active compound IMP-ICCA-9710 inhibitor underwent laboratory testing that simulated operating conditions at which inhibitors need to work.
To indicate protection efficiency, behavior was compared with other commercial inhibitors. Two different inhibitors from different companies were chosen, out of which the best performance were Inhibitor K, a linear-chain amine compound, and Inhibitor AT, a carboxylic acid compound linear-chain. Inhibitor IN3, an imidazoline compound, served as reference inhibitor.
Different methods were used to evaluate the inhib itors, from traditional techniques to more sophisticated ones. The active compound IMP-ICCA-9710 was evaluated in the following tests.
Weight loss
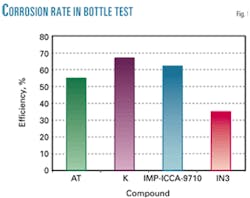
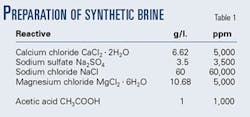
The NACE ID-182 Bottle Test Method is one of the most common tests for corrosion measurement by weight loss. Such a test used synthetic brine (Table 1). The sour acid medium was obtained by use of acetic acid and bubbling H2S to 250 ml/min for 20 min to saturation, resulting a 1,140 ppm H2S concentration.
The test was carried out in 48 hr using a relation of 20% hydrocarbon and 80% brine and carbon steel as testing material. Fig. 1 shows that Inhibitor K presents a higher performance followed by IMP-ICCA-9710.
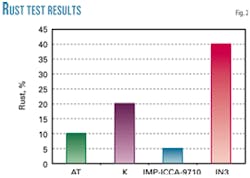
Rust-prevention test
Another test, rust-prevention test NACE TM-01-72, evaluates the efficiency of different inhibitors in preventing rust caused by gasoline and other fuels with water when moving through the pipes and pipelines.
With this test, it is possible to watch for any emulsifying effect the synthesis product might show.
The gasoline in this test was filtered through a column packed with silica (mesh 60-80) before being used. This test lasts about 4 hr in which a bath with temperature control is used to keep a steady temperature (40° C.).
The set amount of gasoline with the corrosion inhibitor (50 ppm) is poured in followed by the coupon, with the mixture being constantly stirred for 30 min; then 30 ml of water are added, stirring until time finishes; at the end of the test the coupons are removed and visually tested.
The test analysis shows that the IMP-ICCA-9710 inhibitor displays the least rust proportion, as it causes the formation of a more persistent film (Fig. 2).
Electrical resistance probe
In the field, the most common methods for monitoring corrosion rate are weight loss and electrical resistance probe (known as "corrosometer"). The former gives monthly averages but it does not give the complete background during the length of the test. The corrosometer yields the corrosion rate on a more moderated basis (each 24 hr).
Accelerating the corrosion rate required a synthetic brine prepared to match pH 4 with H2S, as described:
- Weigh the following reactives and adjust to 500 ml with distilled water to obtain a totally dissolved mixture: NaCl, 30.0 g; CaCl2-H2O, 3.31 g; MgCl2-6H2O, 5.34; and Na2SO4, 1.75 g. Load the reactor with the saline solution.
- Lower the temperature to 0° (±5°) C. with a bath of water and dry ice. As the temperature decreases, the saline solution must be bubbled with nitrogen, for 30 min.
- Add H2S 30 ml/min for about 20 min.
- Measure the pH. In the event pH 4 is not achieved, more H2S must be added. Once pH 4 has been reached, the H2S addition is stopped.
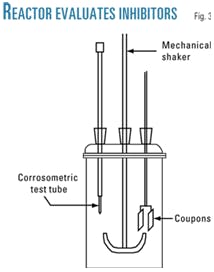
For evaluation of the corrosion rate by use of corrosometer and weight-loss technique, a glass reactor with a 500-ml capacity was made up (Fig. 3).
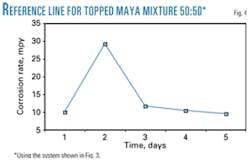
Once the brine was prepared, a reactor was loaded with 400 ml of a topped Maya crude and brine mixture 50:50 (vol %). Fig. 4 shows the corrosion rate during a 5-day period, with an average of 12.5 mpy for corrosometer and 8.5 mpy for weight loss, and the inhibitor effect under these test conditions.
The corrosimetric test tube used was a Rohrbach Cosasco Systems Inc. Model 3000-W40-K03005-24-1-1, and the coupon for the weight loss was made up of carbon steel 1018 with 1.5 in. x 0.5 in. x 0.1 in.
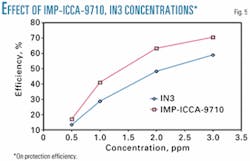
With the topped Maya crude and brine mixture 50:50 (vol %) used as the corrosive medium, IN3 and IMP-ICCA-9710 inhibitors' efficiencies were evaluated in a concentration from 0.5 to 3 ppm. Fig. 5 shows that the IMP-ICCA-9710 corrosion inhibitor gives a higher protection efficiency than the inhibitor currently used in the field, reaching a 10% greater efficiency.
Dynamic circuits test
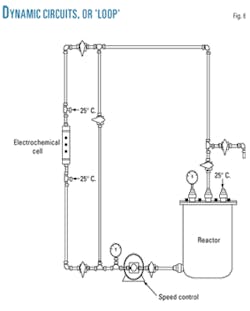
The use of dynamic circuits, also known as "loops," has proven useful in evaluating corrosion inhibitors' efficiency, making it possible to observe performance at any moment during the test (Fig. 6).
This test simulates conditions in pipes and pipelines and allows flow speed, temperature, pressure, corrosive media, specimen geometry, so forth to be modified.
In the present test, corrosion inhibitors'efficiency was determined through such traditional techniques as the weight-loss method and electrochemical techniques by means of a dynamic circuit loop.
In the loop, variables were set as similar as possible to those observed during normal operation: pipe geometry, flow speed, temperature, and evaluation material.
In the case of brine, it is prepared as similar as possible to that found in the field in such a way as to be easy to reproduce to observe in a short time the inhibitors' behaviors. Because hydrocarbons represent a problem in implementing electrochemical techniques, considering their high resistance, it was decided to use only brine. Table 2 presents the loop's operating conditions.
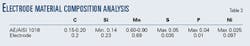
The electrochemical cell electrodes were made up of 1018 steel, confirmed by chemical analysis and verified by its microphotographs (Table 3).
All the electrodes used were mirror-polished and stored in hexane until they were used.
Before brine was loaded into the loop reactor, it was purged three times with nitrogen to avoid the presence of oxygen. Afterwards, the reactor was loaded with 2 l. of brine.
A test without inhibitor was run for 24 hr for each inhibitor. The inhibitor was added later and evaluated over 48 hr.
Fig. 7 presents the corrosion rates for the four inhibitors. As the beginning of each graphic shows, the corrosion rate is very high and decreases until reaching a constant value over 24 hr. This is the time set as blank (without inhibitor). Although repeatability is not high, the protection behavior can be observed when the inhibitor is added.
For K and IN3 cases, a similar behavior is observed: Once an inhibitor is added, a corrosion decrease is observed, but after 24 hr the corrosion rate increases.
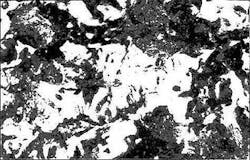
Apparently, this is caused by detachment of the protection film uncovering the metal, as shown by the microphotographs taken of the electrodes after the test was finished (Fig. 8).
IMP-ICCA-9710 and AT samples showed the best protection efficiencies over time.
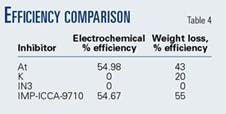
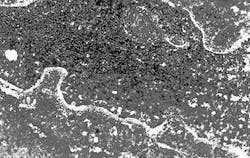
This is verified with the microphotographs in which uniformity on metal surface is observed (Fig. 9). This is also proven when comparing the efficiency percentage with the electrochemical technique and weight loss (Table 4).
The IMP-ICCA-9710 inhibitor presented the best performance both with electrochemical techniques and weight loss followed by the AT inhibitor.
IMP-ICCA-9710 contains an active compound organic type, soluble in hydrocarbon and dispersable in water, containing as a main component an active carboxy-imidazoline type, with a long chain containing from 12 to 20 carbon atoms.
Continuous use of this product, in doses of from 2 to 3 ppm, can control carbon steel pipeline internal wall corrosion. The present formulation showed the highest efficiency in pipe protection in stratified flow because it is water dispersable and permits the active compound to spread through water reaching the metal surface and protecting it from the corrosive agents' attack with a protective film.
Another characteristic of the IMP-ICCA-9710 composition is that the concentrations at which pipes are dosed present no foaming problems. The inhibitor also has a high molecular weight and consequently a high melting point and a high level of thermal stability, as shown in the thermogravimetric analysis.
Bibliography
•Byrne, Norman E., and Johnson, John D., "Water soluble corrosion inhibitor," Patent (US): 5322640.
•Edwards, A., Osborne, C., Webster, S., Klenerman, D., Joseph, M., Ostovar, P., and Doyle, M., Corrosion Science, Vol. 36 (1994), No. 2, p. 315.
•Fisher, L.E., "Corrosion inhibitors and neutralizers past, present and future," NACE Corrosion/93, New Orleans, Mar. 7-12, 1993.
•Kennelley, Kevin J., Thomas, Eugene R., Voorhees, Robert J., Watson, James D., and Sullivan, Daniel S., "Method of Inhibiting corrosion in oil field produced fluids," Patent (US): H1147.
•Larsen, Arthur Lee, Marklund, Soren Johannes, and Rosenbloom, Jan., "Corrosion retarding compositions comprising hydrazine salts," Patent (UK): 2028810.
•Roche, Marcel, "Systematic program and internal inspection keys to corrosion control," OGJ, Apr. 8, 1991, p. 72.
•Maddox, Jim Jr., "Carboxylic acid salts of 1-aminoalkyl-2-polymerized carboxylic fatty acid imidazolines," Patent (US): 3758493.
•Mandke, J.S., "Corrosion causes most pipeline failures in Gulf of Mexico." OGJ, Oct. 29, 1990, p. 40.
•Moldt, Peter, and Nielsen, Elsebet, "Imidazole Compounds," Patent (CA): 2069144.
•Morin, Bruno E., and Goliaszewski, Alan E., "Methods of Inhibiting Water Corrosion in Crude Oil Pipeline," Patent (US ): 2123936.
•Oppenlaender, L., Knut, Stork L., Karl, and Barthold M., Klaus, "Imidazoline based corrosion inhibitors which inhibit corrosion caused by CO2 and H2S." Patent (US): 4388214.
•Pierce, Claudia C., Grattan, David A., and Fong, Dodd W., "Modified polymers incorporating fatty amines." Patent (US): 4999161.
•Roche, Marcel, "Systematic program and internal inspection keys to corrosion control," OGJ, Apr. 8, 1991, p. 72.
The authors
José Luis Benitez Aguilar is a researcher in the corrosion technology group of the Mexican Petroleum Institute. He has a wide range of experience in crude oil processing from corrosion inhibitor research and neutralizers to crude unit overhead. Benitez received a PhD in chemistry from Universidad Autónoma Metropolitana (México).
Claudia Martínez Ortíz is a researcher in corrosion technology of the chemical products department of the Mexican Petroleum Institute. She has experience in corrosion inhibitors research and evaluation for the transportation and processing of crude oil. Martínez received her masters from the National Polytechnic Institute.
Raul Roldan is a chemical researcher working at the Mexican Petroleum Institute. Since 1992, he has participated in the technical support staff for application of specialized chemical treatments in refineries and crude oil facilities; specifically with corrosion inhibitors, emulsion breakers, antioxidants and dispersants. His experience covers 27 years research and development in chemical products for refineries processes. For the last 3 years, he has been responsible for the development of new technologies in chemical products and has been collaborating in molecular engineering projects. Roldan holds a BS in chemical engineering from Puebla University, México.