Pipeline liner material wears well in tests of field specimens
Tests on two field-aged specimens of polyamide-11 (PA-11) pipeline liners in carbon steel crude oil and gas production pipelines indicate the material protects against internal corrosion when chemical inhibition programs, conventional liner materials, or specialty corrosion-resistant alloys are either infeasible or uneconomical.1
Two field-aged specimens were supplied for this analysis by oil field operators. The liners appeared in excellent condition, with residual mechanical properties close to those of the virgin liner.
In each case, the liner was operating without failure, the operating conditions were reasonably well documented, and unexposed reference samples of the recovered liner were available for comparison.
The tests measured polymer molecular weight, residual mechanical properties, and extractable fraction analysis. As the specimens did not age to failure, it is impossible at this time accurately to predict how long the liners will last, although there have been attempts at lifetime prediction.2-4
PA-11 has been used as a corrosion-resistant fluid barrier in flexible pipe for more than 25 years and, more recently, in rigid steel and composite pipes.4-8 Polyamides are generally stable materials that have found wide commercial application.
The chemistry and oil industry-related properties of PA-11 have been discussed elsewhere.9 Based on the ability to withstand more severe operating temperature, pressure, and produced-fluid composition, operators may select PA-11 as the liner material over the more conventional high density polyethylene (HDPE).
PA-11 combines high dimensional stability, low gas-permeation rates, and high tensile strength that makes it suitable for installation as a liner in rigid steel pipes that transport crude oil, gas, and produced water.
It has been installed around the world by all the major liner-installation methods, and liners are commercially available from a variety of vendors. More than 10 km of PA-11 liner in various diameters have been operating in corrosive service pipelines in North America and Europe.
Specimens; test results
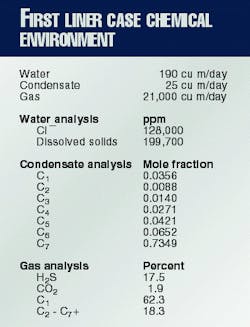
In November 1995, the first PA-11 liner was installed in a rigid steel production pipeline in western Alberta.6 12 It was installed by a roller diameter-reduction method.10 The 4-in. pipeline transported sour gas, gas condensate, and water at about 65° C. and 600 psi.
The pipeline was buried in relatively dry earth so that a nearly isothermal condition existed through the thickness of the polymer liner. Table 1 gives the details of the produced fluid.
The water pH was in the range of 5 to 5.5 in the flow stream. Several HDPE liners had previously been used in this pipeline; the longest service life observed before failure was about 18 months.
At the time of the PA-11 liner installation, a lined spool piece was inserted at the wellhead end of the pipeline with the intent of replacing the spool piece during a scheduled shutdown later to assess the condition of the liner.
This would prevent the premature replacement of the liner in this sour gas line and provide a reference piece to verify the performance expectations held at the time of installation.
The pipeline was shut down for 212 days in various durations from November 1995 to May 1998 due to problems unrelated to the pipeline. During these times, the temperature dropped to the ambient ground temperature, which varied depending on the time of year.
The annulus vents for the first case liner were checked periodically. The annulus pressure never exceeded 25% of liner pressure.11 The PA-11 liner operated successfully through at least one decompression event that, according to the operator, would have collapsed an HDPE liner.
In autumn 1998, during another shutdown, the test piece was removed for analysis. A sample of the original liner had been saved and stored in the laboratory for analysis at the same time as the recovered liner.
Fig. 1 shows both the recovered liner and the reference piece. The recovered liner differs from the reference piece only in color and in the presence of slight surface scratches that resulted from installation. The color difference is due to the absorption of crude production fractions that are slightly soluble in the polymer.
The liner thickness of both specimens is the same as at installation. There is no evidence of collapse or mechanical deformation, although substantial annulus pressures were thought to be present during at least one of the shutdowns due to gas permeation through the liner.
The tensile properties of the aged liner and the retained reference specimen were measured according to the ASTM D-638 standard test method. Table 2 shows the results.
The results are consistent with values obtained in laboratory tests of PA-11 aged in hydrocarbon fluids in which the elongation at break was reduced slightly because of plasticizer extraction.12 The plasticizer is responsible for decreasing internal friction in the polymer.
Polymers owe their ductility and strength to their highly entangled morphology. During elongation under tension, the polymer molecules disentangle up to the point that the molecules are so tightly knotted that disentanglement is disfavored compared to polymer backbone chemical bond breakage.
The plasticizer acts as a lubricant in this process, increasing the elongation (disentanglement) before finally the polymer breaks.
Disentanglement is a process that is affected by temperature and rate. At higher temperatures, the polymer will elongate more before breakage than at lower temperatures. Slower elongation rates also result in larger elongations to break.
The effect on the polymer when the plasticizer is removed, or replaced by a less effective plasticizer, is to reduce the maximum elongation at break at a given temperature and elongation rate.
Table 3 shows the results of the chemical analysis. The biggest difference between the used liner and the retained control is the vacuum extractable fraction. This is a measure of the weight fraction of the material that can be volatilized in high vacuum at a specimen temperature of 230° C., which is above the melting temperature of the polymer.
It can be interpreted as the weight fraction of remaining plasticizer13 and low molecular weight species in the polymer such as adsorbed hydrocarbons. (At the use temperature of 65° C., the equilibrium water fraction in the polymer is about 2.1 wt %. The vacuum extractables do not include this water fraction, which is measured separately according to ASTM Method D-4019-88.)
The produced hydrocarbon fluids normally extract a fraction of the plasticizer. Low-molecular-weight oil or condensate fractions replace most of the extracted volume. This results in essentially no volume change in the polymer liner.14
After correction for low molecular-weight extractables, inherent viscosity is a good index of the molecular weight of the polymer.15 Inherent viscosity is easily measured and requires no complicated equipment or facilities. The results show that there has been a small decrease in molecular weight of less than 10%.
The most complete and accurate measure of molecular weight (although much more complicated and difficult to do correctly) is obtained by measuring the molecular weight distribution by gel permeation chromatography,16 the results of which are shown in the last two columns of Table 3.
The weight average molecular weight (Mw) of the polymer as measured by gel permeation chromatography (GPC) has not changed significantly, as the method is accurate to plus or minus 3-5%. Based on the results shown, it appears that the liner is in good health at this stage in its life.
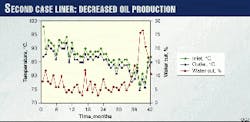
The second specimen set consists of a section of liner that had been in service for about 3 years conveying crude at an average inlet temperature of about 87° C., an average outlet temperature of about 85° C., and an unexposed section of pipe from the same production lot. The liner resided in the host pipe in a neutral stress state. The pH is unknown.
This pipe was operated in an environment that, combined with the low thermal conductivity of the polymer, allowed a thermal gradient to be maintained across the liner wall thickness.
The presence of the external heat sink also created a thermal gradient along the length of the pipe as the produced fluid cooled while flowing through the pipeline. During the course of operation, the water cut rose from slightly less than 5% to more than 15%. Fig. 2 shows the water cut and temperature history.
Analysis of the PA-11 liner was possible at the cool end, the middle, and the hot end of the pipe, and through the thickness from inside (hot) to outside (cool) surfaces. The unexposed pipe segment was stored in a warehouse during the 3 years that the installed segment was operating in the field. All specimens were analyzed by the same techniques as described for the first pipeline liner.
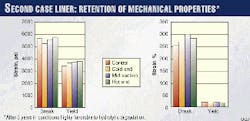
Fig. 3 summarizes the measured tensile properties. The tensile bars were die-cut from the liner around the hoop direction. There has been very good retention of tensile properties.
Elongation at break, a good indicator of polymer health, has not changed significantly compared to the reference piece. The apparent trend in stress at break and yield from cold end to hot end cannot be explained in the context of the remaining tensile data.
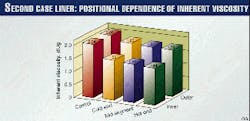
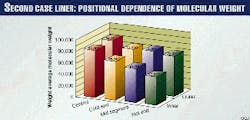
Fig. 4 presents the inherent viscosities, corrected for vacuum extractable fraction. There is evidence of a minor reduction in molecular weight from inside to outside and from cold end to hot end, within each series and compared to the control.
The weight average molecular-weight measurements by GPC (Fig. 5) corroborate the inherent viscosity evidence of a small amount of degradation and that the degradation is a function of temperature.
Aging in service
All polymers can degrade under the right conditions of chemical environment, heat, and radiation. Combinations of these stresses can be particularly aggressive. PA-11 can degrade by any of three basic mechanisms: hydrolysis, thermo-oxidation, and thermolysis. The dominant degradation mechanism is determined by the chemistry of the polymer.
For PA-11 in a petroleum environment, hydrolysis is the dominant degradation mechanism. The mechanism requires water and is accelerated by acids and elevated temperatures.
Hydrolysis results in shorter polymer chains that are less entangled. The entanglement of polymer molecular chains is the reason for the high strength and elongation properties of polymer materials.
Serpe has proposed that hydrolysis is reversible if the water can be removed and the chain ends do not migrate.17 While this is feasible in the laboratory, it has yet to be demonstrated in field conditions. Hydrolysis should then be considered to be, for all practical purposes, irreversible.
Thermo-oxidation requires oxygen and high temperatures. Stabilizers added to the polymer during manufacture retard its rate. This mechanism has yet to be shown to be a significant contributor to degradation of PA-11 in oil and gas pipeline service. This is because oxygen is relatively scarce in the transported products.
Thermolysis, caused by extremely high temperatures, occurs under conditions of very low water and oxygen availability. The rate is so slow at temperatures less than the melting point that it can be ignored as a factor in the performance of operating pipe liners.
Studies of hydrolysis
The hydrolysis reaction that is the primary degradation mechanism for PA-11 has been studied in El Atochem laboratories under conditions of deionized, deoxygenated water at various pH levels. The pH was adjusted with mineral acids.
Injection molded tensile bars were placed in an autoclave and aged in water at various highly elevated temperatures to accelerate the degradation rates. At time intervals, some bars were removed and tensile properties measured according to ASTM D-638 to determine the effect of exposure on elongation at break.
The point in time at which the elongation at break is reduced by half was defined as the half-life in elongation.
The usual tensile elongation at break for PA-11 exceeds 300%. This corresponds to an elongation at break of about 150% at the half-life in elongation. The logarithm of time at a given temperature for the polymer to reach its half-life in elongation is plotted against the reciprocal of the temperature (Kelvin) at which the sample was aged.
The resulting straight line permits prediction of the half-life in elongation, by extrapolation and interpolation, for temperatures not measured. Fig. 6 shows the results of this study.
The effect of pH is not surprising. It is well known that hydrolysis of amides is accelerated by acids and to a lesser extent by bases. These results are not a lifetime model, but they form a basis for understanding the effect of the production environment on the mechanical properties and molecular weight of the liner.
A recent study of PA-11 hydrolysis-dominated aging used inherent viscosity, a measure of molecular weight, to follow the aging process.3 The study independently confirmed the observation that hydrolysis is a thermally activated process, follows classical first-order chemical kinetics, and can be described by the Arrhenius law.
The study further suggested some criteria for definition of end-of-life depending on the safety margin demanded by the application. By the time the inherent viscosity reaches 1.0 through the thickness of the polymer specimen, the elongation at break is reduced to about 50%, from the initial 350% or so.
At this point the polymer still has enough residual strength to operate, but the margin of safety for some high-risk applications may be uncomfortably thin.
Implications
Liners in rigid steel pipelines will provide the intended protection to the steel as long as there is fluid containment and the chemical barrier properties are intact.
In the absence of third-party damage, loss of containment could result from ductile failure caused by collapse pressure in the annulus, from fracture of the liner due to molecular degradation and an imposed strain from annulus pressure, or from chemical degradation to the point of dissolution.
A ductile failure is highly unlikely considering the very high strains needed to cause rupture. Steel pipeline liners are not normally subject to strains of more than a few percent during operation. Correct operation of a PA-11 liner can minimize the probability that annulus pressure will cause a loss of containment until the liner is in an advanced state of degradation.
Failure by dissolution of the liner is unlikely. This requires long periods of time at extreme conditions. It is more likely that loss of containment due to brittle fracture of an extremely degraded liner would long precede dissolution, and this would be detected during the periodic vent checks that are required by government regulations and industry recommended practices.
In any case, the environmental risk associated with liner failure is low because a lined steel pipe provides double containment and the vent checks provide a periodic assurance of liner integrity.
Irreversible collapse of the liner is the dominant operating concern for lined steel pipelines. It is caused by the build-up of high-pressure gases in the annulus between the liner and the steel wall which expand when the line pressure drops, increasing the pressure differential across the liner. This build-up is caused by permeation of gaseous species through the liner.
If, at operating pressures, the annulus volume is large enough and the pressure is high enough, the expanded gas volume could be sufficient to deform the liner severely after depressurization of the pipeline.
If the liner is insufficiently elastic to recover its original shape after repressurization, the collapsed state may impair pipeline operation. Even so, small liner deformations should be expected during the course of operations due to normal operating pressure variations.
The pressure required to collapse a liner fully encased by a steel host pipe increases with the modulus of the polymer and depends upon a variety of geometric factors.18 The modulus of PA-11 increases as the plasticizer is extracted or replaced by light hydrocarbons,12 which increases the liner's resistance to collapse.
Over extended periods of operation in gas-condensate service, the modulus is likely to stabilize at an intermediate value between that of the fully plasticized PA-11 and the unplasticized PA-11, resulting in a greater collapse resistance during long-term use than upon initial installation.
Although no accurate predictions can be made about the time left to end of life, PA-11 liners that were the subject of this study clearly remain suitable for the service in which they were installed.
The first-case liner has lasted more than twice as long as the previous HDPE liners and shows no signs of fragility. The second-case liner also has near-new mechanical properties that make it still suitable as a corrosion-resistant barrier in oil field service.
The first liner has operated for more than three times the economic break-even period at the time of analysis and a quarter the cumulative cost of corrosion inhibition over the same time period.19 Economic data for the second liner were not made available.
The longer term effects of aging in service will be followed for the first test case pipeline. There is another test spool in that pipeline which will be removed after at least another 3 years of service.
The specimen removed for this study was replaced by a new lined spool that can be removed in the distant future.
References
- PA-11 is sold as Rilsan, manufactured by Elf Atochem North America Inc., Philadelphia.
- Dawans, F., Jarrin, J., Lefevre, T., and Pelisson, M. "Improved thermoplastic materials for offshore flexible pipes," 1986 Offshore Technology Conference, OTC No. 5231.
- Jarrin, J., Driancourt, A., Brunet, R., and Pierre, B., "Durability of polyamide-11 for offshore flexible pipe applications," MERL Conference: Oilfield Engineering with Polymers, Westminster, London, October 1998.
- Recommended Practice for Flexible Pipe, API RP 17B, Section 5.5.2, API, 1997.
- Dawans, F., Jarrin, J., Lefevre, T., and Pelisson, M., "Improved thermoplastic materials for offshore flexible pipes," Offshore Technology Conference, 1986 Offshore Technology Conference, OTC No. 5231.
- Lebsack, D., and Hawn, D., "Internal pipeline rehabilitation using polyamide liners," Materials Performance, Vol. 37 (1998), No. 3 , pp. 24-27.
- Stringfellow, W., "Development of an advanced composite spoolable line pipe," NACE Northern Area Western Conference, Calgary, March 1999.
- Kalman, M., and Loper, C., "Development of composite-armored flex risers for deep water" World Oil, November 1998, pp. 103-06.
- Mason, J., "Pipe liners for corrosive high-temperature oil and gas production applications," Materials Performance, Vol. 37, No. 9 (1998), pp. 34-40.
- Titeliner by United Pipeline Systems, Durango, Colo.
- Lebsack, D., "Pipeline liner design and operating experience for sour gas pipelines," NACE Northern Area Western Conference, Calgary, March 1999.
- Mason, J., "Pipe liners for corrosive high-temperature oil and gas production applications," Proceedings of NACE Corrosion 97, No. 80, pp. 80-84.
- The plasticizer in Rilsan polyamide-11 for liners is N-n-butylbenzenesulfonamide. It is present at about 14% and boils at approximately 314° C. at atmospheric pressure.
- Baron, J.J., Szklarz, K.E., and Macleod, L.C., "Nonmetallic Liners for Gas/Condensate Pipelines Joint Industry Project," Final Report, Shell Canada Ltd., 1997.
- Billmeyer, Fred W. Jr., Textbook of Polymer Science, 3rd ed. (New York: John Wiley and Sons, 1984), p. 208.
- Billmeyer, p. 214.
- Serpe, G., Chaupart, N., and Verdu, J., "Molecular weight distribution and mass changes during PA hydrolysis." Polymer, Vol. 39 (1998), pp. 6-7.
- Svetlik, H.E., "UHMWHDPE Liners: An engineered solution to production corrosion problems," Proceedings of NACE Corrosion 85, No. N136. Lo, K.H., and Zhang, J.Q., "Collapse Resistance Modeling of Encased Pipes," Buried Plastic Pipe Technology: Vol. 2, ASTM STP 1222, ASTM, Philadelphia. Kyriakides, S., and Youn, S.K., "On the Collapse of Circular Confined Rings Under External Pressure," International Journal of Solids Structures, Vol. 20 (1984), No. 7, pp. 699-713.
- Lebsack, D., BP-Amoco, private communication.
The Author
James F. Mason is technical manager for oil projects, Elf Atochem North America, Birdsboro, Pa. He joined Elf Atochem in 1987 and has been involved in engineering polymer applications for 18 years.
Mason holds a BA (1979) in chemistry from State University of New York, Potsdam, and is a member of NACE and SPE. Within NACE, he chairs the task group T-10E-10 on thermoplastic liners for oilfield pipelines.