Formation mineral content key to successful sandstone acidizing
To improve the success of sandstone-acidizing, operators must know the mineral content of producing formations. Otherwise, they may encounter problems with clay swelling, fines migration, gel formation, and precipitation.
Both laboratory research and field examples show the need to tailor treatments to formation minerals.
Ion exchange, clay swelling
Ion exchange occurs between formation minerals (usually clays) and injected brines. Sodium ions are often attached to these clays, but these ions can become exchanged with ions of the injected fluid.
When ion exchange occurs, the clays convert the injected fluids to salt water of the corresponding concentrations. For example, when 3% NH4Cl (ammonium chloride) flows across an ion-exchanging clay, the solution becomes 3.3% NaCl (sodium chloride) brine. Exposure to this low-salinity brine can cause water-sensitive clays to swell and obstruct matrices.
Table 1 lists some minerals and rates their capabilites to exchange ions and to transform brines. The rating of a mineral is directly proportional to its ion-exchanging capability. Sand, feldspars, and kaolinite have zero ratings.
Of the ion-exchanging clays listed, smectite and mixed layer (illite and smectite) clays are the most water sensitive and hence most prone to swelling after ion exchange.
Operators who identify or suspect the presence of these clays in their formations need to use brines that transform into about 6% salt water after ion exchange.
Fluids such as 5% NH4Cl, 7% KCl (potassium chloride), and 5% CaCl2 (calcium chloride) will transform into about 6% salt water after ion exchange.
Consequently, such fluids have concentrations high enough to maintain formation permeability by preventing clay swelling. By contrast after ion-exchange, 2% KCl will transform into a 1.5% salt water, a brine too weak to prevent clay swelling.
Zeolites have posed problems for fields in the U.S. Gulf Coast. Zeolite minerals will not swell but have very high ion-exchange capacities.
A formation containing 10% zeolite can have the same ion-exchange capacity as a formation containing 40% smectite. Such minerals are very likely to contain sodium that could contaminate spent HF (hydrofluoric) acid, or these minerals can very quickly transform 2% KCl into a weak salt water.
Clay swelling also becomes a problem when operators do packed-fracture (frac-pack) treatments without analyzing formations for water-sensitive minerals. The following case history illustrates this trend:
A well operator contacted a service company, saying that an onshore frac-pack job had killed a well. The operator had X-ray analysis showing 20-30% mixed-layer clays at the site, and clays tend to swell during deep matrix invasion.
To test this theory, the operator asked to treat another well with a formation makeup similar to that used in the killed well. But in this second well, the frac pack contained 7% KCl fluid.
Because 2% KCl is commonly used for this kind of frac-pack job, the service company had to convince the operator that 7% KCl was needed to produce a brine heavy enough to prevent clay swelling after ion exchange. With this fluid the frac-packed well responded properly.
This field test helped confirm that understanding the minerals contained in a formation is important for designing any fluid treatment. Also, operators must use injected brines that are compatible with water-sensitive formations before and after ion exchange.
Including polymeric clay stabilizers is another strategy operators can use to prevent clay swelling in water-sensitive formations. These stabilizers render clays nonswelling even to freshwater and should be used to treat the critical areas that are 2-3 ft from the well bore.
Fines migration
Fines migration is a production problem that can be minimized by tailoring stimulation treatment to a formation's minerals.
Most fines migration problems result from the physics of flow and involve two kinds of clay: kaolinite and illite. Both clays are loosely bound and can be easily dispersed by production velocities near the well bore.
Once the fines leave the matrix, they usually flow unobstructed through perforation tunnels and gravel packs. These fines cannot be pushed back into the reservoirs by stimulation treatments.
They can be pushed to the sides of the pores they are plugging, but such effects are short-lived. Reperforating can temporarily succeed, but the production will continue to decline faster than depletion would predict.
The best way to deal with fines migration is to identify the minerals in the formation. If kaolinite and illite are detected in X-ray analysis, operators can use a high perforation density, acidize a clean zone, and apply a polymeric clay stabilizer.
A high perforation density (8-12 shots/ft) distributes flow velocity to help minimize the severity of fines migration.
Operators can also dissolve the fines that are close to the well bore by pumping about 100 gal/ft of HF acid. This volume, however, will clean the clay and feldspars from only a fist-sized volume of formation rock at each perforation.
Polymeric clay stabilizers may be used to stabilize the remaining near well bore fines.
These approaches will not eliminate all fines migration but will certainly reduce their severity. Many operators prefer to pump only 20 gal/ft of HF acid/perforation, but this will clean only a very small volume of rock, leaving fines very close to the perforation face.
A treatment of 20 gal/ft/perforation is likely to clean only the thumb and forefinger of the fist-sized volume of rock. This is all the dissolving power that such a volume can provide. Still, the fluid will penetrate several feet into the formation.
Clay stability
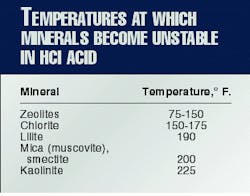
Clay decomposition in HCl (hydrochloric) acid is a recent, important discovery in formation mineralogy. Many operators already know that chlorite is not stable in acid. What they may not realize is that all clays have temperatures above which they are unstable in acid.
Table 2 lists the temperatures above which some minerals should be considered unstable in HCl acid.
Zeolites are very unstable in acid. Some zeolites will decompose in acid even at room temperature.
Halliburton researchers pumped HCl acid into a long column with illite dispersed in the pack and were surprised to find that the illite decomposed, precipitating silica gel.
The concept of soaking formations with HCl acid should be reevaluated. Data in Table 2 indicate that any acid soak at 275° F. with HCl acid will turn most clays into jelly. It is a wonder that operators have had any success in the past with HCl-acid soaks on sandstone formations.
For example, if an operator pumps 15% HCl acid into sandstone that does not have carbonates and shuts in the acid for 2 hr at 200° F., no acid will be recovered. The spent acid only will be recovered in the form of water.
The first portion of the recovered spent acid could have a pH of 1. Most of the acid, however, will have decomposed the clays in the formation, thereby consuming the HCl.
Recent research has also provided an understanding of the nature of the plugging problems caused by clay instability. Understanding the nature of these plugging problems can help operators design effective treatments.
For example, many operators already know to watch out for chlorite. When core reports show chlorite present at 0.5-2%, operators know that to avoid precipitating iron, they should not use HCl acid.
But iron is not really the problem. When chlorite is acidized by HCl acid, it does release iron (and other clays will release sodium or potassium). The plugging problems are not caused by iron, however, but by silica gel that forms, polymerizes, and creates plugging, colloidal particles.
Hence, iron control additives will not make an acidizing treatment more effective because these additives do not address the chemical nature of the plugging problem.
Operators can avoid these plugging problems by using alternative acid treatments in sandstone formations. Clays are stable in acetic acid and formic acid.
Unfortunately, clays react to acetic acid as though it were freshwater. Consequently, acetic acid does not decompose clays but instead causes clays to swell. In the past, acetic acid had been used unsuccessfully in sandstone formations that contain zeolites.
The Gulf of Mexico region, in particular, has problem formations that contain zeolites and swelling clays that swell on contact with acetic acid. Acetic acid also has caused swelling of clays containing smectite and illite.
As another example, when service company researchers pumped acetic acid into a column containing sand and smectite, the column "locked up" after the acid penetrated just a few inches into the column.
This problem of clays mistaking acetic acid for water can be solved by addition of 5% ammonium chloride to 10% acetic acid. This produces a fluid that will help prevent decomposition and clay swelling after ion exchange is complete.
Operators must remember that:
- Acetic acid cannot tackle the problems of sulfide and iron scales.
- Operators cannot "pickle" their pipes with acetic acid.
- HCl acid must be used to remove sulfide and iron scales.
Mineral reactions
Knowledge of formation mineralogy can also help operators predict which minerals are likely to form matrix-blocking precipitates during sandstone acidizing treatments.
The three main mineral groups that will produce significant precipitates upon contact with HF acid are: sodium-containing minerals, potassium-containing minerals, and carbonates.
Sodium-containing minerals will precipitate silicon fluorides 6-18 in. from the well bore.
Potassium-containing minerals will also precipitate silicon fluorides but closer to the well bore and in greater quantities. The silicon fluorides produced by potassium-containing minerals have more plugging capability than the silicon fluorides produced by sodium-containing minerals.
Carbonates will precipitate calcium fluorides upon contact with live HF acid. Calcium fluorides, however, are not the problem. The main problem with carbonates is that they will precipitate aluminum fluorides as spent HF acid invades the matrix.
The industry became aware of this problem in the late 1980s when some well bores became plugged with aluminum scales.
If aluminum scales can deposit in the well bore, they can also deposit in the matrix.
Most sodium problems occur when HF fluids react with sodium feldspars, ion-exchanging minerals, and formation brines. Sodium feldspar has only recently been singled out as a source of precipitation problems.
In the late 1970s and early 1980s, X-ray analysis did not generally distinguish between specific types of feldspar. Today, however, powder diffraction X-ray equipment can produce digitized spectra analyses that allow determination of which kind of feldspar is present.
Precipitated salt from completion fluids is another source of sodium.
Heavy completion fluids such as calcium chloride and calcium bromide, both of which are kill fluids, will precipitate salts out of formation waters. The plugging problem becomes particularly acute when operators inject kill fluids into formations containing saturated salt waters and then try to bring the kill fluids back.
Because the formation waters cannot tolerate any more sodium, the salts crystallize out, leading to scaling and plugging problems. A 15% acid treatment will also precipitate salt out of saturated salt water.
The major sources of potassium are potassium feldspars, mixed-layer (illite and smectite) clays, micas, and KCl kill fluids.
Potassium feldspars have the greatest potential for causing failure of typical HF-acid treatments. Unfortunately, potassium feldspars are usually the most abundant.
The most commonly-known carbonates are calcite and dolomite. There is, however, a full range of carbonates whose structures contain various amounts of calcium, magnesium, and iron.
Ankerites and siderites are carbonates that dissolve very slowly in HCl acid at 150° F. Ankerites and siderites, however, will dissolve more quickly if fluoride is present in the acid, as in spent HF acid.
The North Slope of Alaska has problem formations containing clays and siderites. If such clays are subjected to HF acid treatments, the spent HF acids flow across the siderites, forming aluminum precipitates that plug formations.
Sandstone formations containing more than 5% carbonates are prone to matrix precipitation of complex aluminum fluorides, as spent HF acid flows across the carbonates. As a result, it is important to design the acid preflush so it removes carbonates for a distance of at least 2 ft from the well bore.
Operators of wells in formations that have 8% carbonates cannot pump enough acid preflush to remove such carbonates satisfactorily.
At least 500 gal/ft of 15% HCl preflush is needed to remove 8% carbonate for a distance of 5 ft from the well bore. It requires only 100 gal/ft of 15% HCl preflush to remove 8% carbonate for a distance of 2 ft from the well bore.
Operators must realize, however, that even if there is 5% carbonate in the matrix at a distance of 2 ft from the well bore, the HF acid pumped into the matrix will eventually react with the carbonate.
Consequently, operators must be ready to pump adequate volumes of acid preflushes into matrices that contain carbonates.
Preventing precipitation
Problems with precipitating minerals are best avoided by identifying the formations' minerals and designing acid treatments that are compatible with these minerals.
Sodium precipitation can be avoided through the use of preflushes that not only dissolve brines and salts but also wash them off the surfaces of ion-exchanging minerals.
For carbonate removal, acetic acid can be used, and as a rule of thumb, it is recommended that this acetic acid treatment be at least equal in volume to that of the HF acid stage.
A general conclusion that one can draw from all these precipitation problems is that the use of 12% HCl-3% HF mud acid as the first choice in acidizing treatments should cease, unless an operator has very clean sand (containing 90-100% quartz) or silica scales.
If the sand is very clean, 12% HCl-3% HF mud acid is a good place to start. But if formations do not contain very good clean sand or silica scales, operators should start with 13.5% HCl-1.5% HF acid.
If potassium feldspars are present in the formation, a 9% HCl-1% HF acid mixture is preferred to assist in avoiding plugging problems.
To protect spent HF acids from fluids coming behind them, operators should use overflushes that displace spent HF acids to about 2 ft from the well bore, so that reactions do not occur in the near well bore region.
Sandstone acidizing future
Simulation of downhole plugging in the laboratory has increased the understanding of the minerals that cause problems at the well site.
Lab results show that the best way to avoid these problems is to know the formation's mineral content (Table 3). Based on the kinds of minerals, operators can choose the correct acid treatment and can predict potential problems between these minerals and injected fluids.
The current state of technology might permit acid treatments at temperatures up to 375-400° F. In such conditions, formic and HF acid solutions as well as acetic and HF acid solutions will not work well. They will instead precipitate aluminum trifluorides just outside the fist-sized volume of cleaned rock around each perforation.
As recently as 1990, the author advised five Gulf Coast well operators not to use acid treatments. The recommendation was made with the knowledge that eventually fines migration in formations in this region would force these operators to opt for acid treatments, after exhausting other remedial measures.
With current technology, use of treatment fluids that do not cause clay swelling could enable the operators to avoid damaging their formations and the consequent need to apply acid for damage removal.
As late as 1990, many people in the industry considered the average success rate for HF acid treatments to be 20-25%. Acid treatments are cheaper than other alternatives, and consequently operators have no qualms about using acid treatments in spite of their high failure rate.
In contrast, if frac-pack treatments enjoyed a success rate of only 20%, frac-pack companies would have gone out of business long ago. The prevailing tolerance for a high rate of failure of acid treatments should end.
To minimize risks, operators and acid-treatment specialists must know something about the minerals in formations before pumping acid into the well. Present-day technology can boost the success rate of acid treatments.
Recently, an organic acid/HF fluid was used successfully in four wells in Thailand, boosting production to 1,000 b/d from about 100 b/d. The success was attributed to the avoidance of precipitation problems.
To help avoid inducing formation damage as a consequence of applying acid treatments, operators should follow these steps:
- Determine what minerals are present in the formation from historical records, X-ray analysis, or spectral gamma ray log.
- Establish ion-exchange rates of clays and use brines that are compatible with swelling clays before and after ion exchange.
- Use 10% acetic acid with 5% NH4Cl in acid-sensitive formations, so that clays do not turn to silica gel.
- Avoid HCl acid "soaks" on sandstone formations.
- Use proper brines for deep matrix invasion.
- Check the compatibility of HF acids with formation minerals, and protect spent HF acid.
- Remember that feldspars and clays contain sodium and potassium, and that carbonates cause aluminum fluorides to precipitate and plug up matrices.
- Take care when gathering samples to ensure that they are truly representative of the entire formation.
The Author
Rick Gdanski is a scientific advisor in the production-enhancement section at Halliburton's Duncan technology center. His responsibilities include research, teaching, and treatment design in the areas of sandstone acidizing and carbonate acidizing.
Gdanski holds a BS in chemistry from Southwestern Oklahoma State University and a PhD in physical organic chemistry from the University of Illinois.