In-depth phase characterization improves naphtha cracker emulsion breaking
Fabrice Cuoq
Jérôme Vachon
Saudi Arabian Basic Industries Corp.
Geleen, the Netherlands
Research by Saudi Arabian Basic Industries Corp. (SABIC) shows that colloid chemistry analytical tools combined with advanced analytics enable clearer understanding of the emulsion properties. Dynamic surface tension as well as zeta and streaming potential titrations yield valuable information on the molecular size, electrostatic charge, and isoelectric point (IEP) of the emulsifier. These combined, measured parameters can be used to determine the emulsion-stabilization mechanism, which in turn allows a proper and tailored optimization of plant conditions.
Background
Naphtha-cracking processes often suffer from oil-in-water (OW) emulsions in quench water towers (QWT). Organics within the water phase can deposit in the dilution steam generators (DSG) and cause severe fouling that can lead to critical energy losses and threaten production. In practice, emulsion-breaker dosage or pH reduction are commonly used to break these emulsions. The mechanism of emulsion stabilization via emulsifiers, however, is often unknown due to the complex composition of both aqueous and gasoline phases.
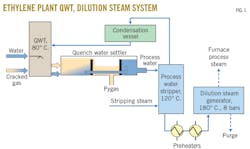
An ethylene plant’s cracked gas needs to be cooled before proceeding to the cracked-gas compressor section. This is generally achieved by quenching the cracked gas with a water stream in a QWT. The QWT is generally followed by a quench-water settler tank that allows water and pyrolysis gasoline (pygas) separation. The pygas usually contains a mixture of light hydrocarbons such as benzene, toluene, styrene, ethylbenzene, cyclic hydrocarbons, and C5-C10 unsaturated components. This stream is commonly recovered after the QWT for further treatment, and the water phase is sent to a process-water stripper (PWS) column to remove dissolved hydrocarbons. Depending on plant design, condensates from the top of the PWS can be rerouted to the QWT as quenching water or sent to the water treatment plant. The bottom of the PWS is finally routed to the DSG, where process steam is produced for use as diluent in the furnaces. The system from the QWS to DSG is called the dilution steam system (DSS) (Fig. 1).
Either OW or water-in-oil (WO) emulsions can form in the first compartment of the QWT and may lead to carryover of process water or pygas to sections not adapted to treat such effluents, which can lead to severe issues impacting plant economics, including corrosion, formation of polymeric deposits, or in extreme cases, an unplanned shutdown of the petrochemical plant. OW emulsions are generally the largest cause for concern. Solid fouling deposits—which result from the high reactivity of pygas—can be formed in the DSS, which subsequently leads to severe energy capacity losses in PWS, preheaters, and the DSG.
In practice, emulsion-breaker dosage or pH reduction are used to break these emulsions. Physical separation methods such as coalescers can also be applied. If missing, however, this solution requires a high-cost hardware change to the cracker.
Despite the different existing solutions, the mechanism of emulsion stabilization and the structure of the emulsifier(s) are often unknown due to the complex composition of both phases and the high variability of feedstocks cracked in an ethylene plant. Empirical solutions are often developed for these systems by using simple shake-bottle tests without investigating an emulsion’s true nature and properties.
To our knowledge, this is the first time that colloid chemistry analytical tools have been applied to resolving OW emulsion issues in a naphtha cracker. The results presented in this article have all been obtained using real plant samples from one of SABIC’s European naphtha crackers.
Materials
Four OW emulsion samples (pH = 8.8, σ = 135 microsiemens (μS)/cm) were taken at different times from a QWS at one of SABIC’s naphtha crackers. The milky-colored water samples—which indicate dispersed organics in the water—were obtained from the bottom of the gasoline-water separator vessel (Fig. 2).
Samples of OW emulsion taken from the naphtha cracker’s QWS were milky colored, indicating the presence of dispersed organics in the water (Fig. 2).
Methods
A SITA science line t-60 maximum bubble-pressure tensiometer measured dynamic surface tension. Streaming potential titration determined the charge density of the emulsion. Measurements were performed on a Mütek PCD03PH streaming potential detector. A total of 19.81 g of process water was titrated with a polydiallyldimethylammonium chloride (PolyDADMAC) (1 milliequivalent (meq)/l.) organic coagulant. While 0.282 ml of the PolyDADMAC solution was needed to neutralize all charges in the process water, the titration of the cell itself required 0.220 ml of the blank solution.
The pendant-drop method determined interfacial tensions. In this method, the shape of a droplet suspended from a needle in a nonmiscible bulk-liquid phase is determined. The interfacial tension can be derived from the drop shape and the density difference between the two phases. Analysis was performed with an Attension CAM-200.
Average gasoline-droplet size was measured using a Malvern Mastersizer 2000, with the mean diameter (volume-weighed mean) of the droplets found to be about 11.08 μm.
Salting out the process water (NaCl 100g/l.) measured the amount of gasoline dispersed in the water. Once the emulsion was broken, the amount of phase-separated gasoline was measured volumetrically, revealing that the process water contained about 0.1% of dispersed gasoline.
Zeta potential values were obtained with a Malvern Zetasizer NanoZS at 70º C., and the emulsion’s pH was lowered using a 0.1 volume-to-volume (v/v) % HCl solution.
DSM R&D Solutions BV, Geleen, the Netherlands, measured liquid chromatography-mass spectrometry (LC-MS) on a Bruker Daltonics maXis Quadrupole Time-of-Flight (Q-TOF) mass spectrometer with electrospray ionization (ESI), atmospheric-pressure chemical ionization (APCI), and sometimes atmospheric-pressure photoionization (APPI) in the positive and negative-ion mode. The elemental compositions of the visual peaks were manually determined based on the accurate mass.
Direct insertion probe-mass spectrometry (DIP-MS) was performed by DSM Resolve on a Waters Autospec sector-field mass spectrometer.
Discussion
Zeta potentials (ζ) of -80 ± 6 mv (pH = 8.8, σ = 135 μS/cm) were obtained for the emulsion at both 25º C. and 70º C. (average values of the four samples), indicating that the electrostatic component is relatively strong since ζ < -30 mv are generally representative of stable emulsions. Using titration, the charge density of the emulsion was found to be about 2.7 μeq/l. at pH 8.8. By considering an 0.1 v/v % OW emulsion (volumetric value determined by salting out the emulsion) and an average spherical-droplet size of 11.08 μm, the charge density of the emulsion was calculated at 0.62 μeq/sq m.
This calculated value seems low compared to the charge density generally considered for a stable emulsion. While the low charge density and the highly negative zeta potential seem contradictory, they can be partially explained by the low conductivity of the continuous phase (i.e., 135 µS/cm), which could be indicative of the presence of low amounts of adsorbed counter ions. It can finally be concluded that the electrostatic component of the emulsion is not negligible in the specific conditions of the naphtha cracker’s QWT studied. Furthermore, zeta potential measurements can be used to optimize the emulsion-breaker dosing rate in process water. For this, dynamic zeta potential measurements upon emulsion breaker addition can be recorded offline or monitored online.
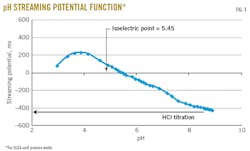
• Determination of IEP and its relation to emulsion destabilization. Recording the titration curve (streaming potential vs. pH) while titrating the emulsion with HCl (Fig. 3) determined its IEP.
From the process water’s original pH of 8.88 to pH 3.45, the streaming potential shows a continuous increase from about -400 mv to +200 mv. The charge inversion (at streaming potential = 0 mv) is obtained at pH 5.45, which corresponds to the IEP. At pH values above the IEP, the droplets are negatively charged, while below the IEP, the droplets are positively charged. In this case, cationic charges are generally caused by amines, whereas anionic charges are generally due to carboxylic acids. Around the IEP, the emulsion is not charged and becomes very unstable. Determination of the IEP will therefore indicate the system’s optimum pH.
Fig. 4 shows the process water at different pH values after 24 hours.
Process water at higher pH values after 24 hours increased in murkiness (Fig. 4).
At pH < 3.86, the streaming potential decreases again slightly because of either the compression of the double layer or a systematic decrease in streaming potential, both induced by the high concentration of ions.
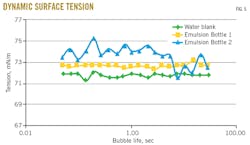
Recording dynamic surface tension measurements allowed estimates of the time needed for the emulsifiers to diffuse to a newly created hydrophobic surface (Fig. 5).
Surface tensions of both emulsion bottles (measured in duplicate) lie well above the ones for pure water, indicating that no free-surface active species are present in the process water. Also, the stable high surface tension indicates that the emulsion is not stabilized by small molecules but rather by amphiphilic polymers because these do not have time to migrate from the emulsion droplet onto the bubble surface for a bubble life of even 10 sec. The fact that the values are slightly above the pure-water value is because the samples were colder (18° C.) than the water reference (25° C.), giving them a slightly higher ionic strength. Surface tension generally increases with lower temperatures and increased ionic strength.
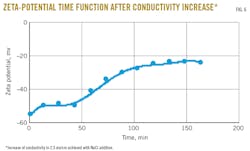
Fig. 6 shows the evolution of the zeta potential as a function of time upon an increase of the conductivity to 2.3 ms/cm (achieved by NaCl addition).
Upon addition of a high concentration of NaCl, the zeta potential starts to slowly increase (become less negative), finally reaching a plateau value of -20 mv. This observation is unusual as zeta potential—like other electrostatic effects involving small ions—is generally rapidly affected within seconds or minutes once the conductivity of the continuous phase is changed. Once the salt solution is added to the system, however, molecular rearrangements at the droplet surfaces occur, causing this slow increase in zeta potential and showing that the thermodynamic equilibrium has not yet been reached. This last observation suggests that the charged groups are attached to polymeric species for which structural rearrangements take place at time scales of minutes to hours.
For a polymeric species to be surface active, it should possess an amphiphile character, meaning that part of its chain must be in the aqueous phase (hydrophilic side) and part of it in the oil phase (lipophilic side). The presence of molecular-chain segments in the aqueous phase implies that steric phenomena play a role in stabilizing a surface. The combined action of electrostatics and sterics is generally called electrosteric stabilization. Based on the presented data and observations, we can conclude the emulsion is electrosterically stabilized.
• Analytical chemistry for emulsifiers identification. Results of earlier testing showing the emulsifier as charged with a polymeric nature prompted selection of the freeze-drying method to isolate and analyze chemical structures of the emulsifiers. Freeze drying consists of freezing the main water phase and sublimating the ice under vacuum. This treatment method evaporates water and light compounds (i.e., volatile monomers) from the dispersed gasoline, leaving only heavier species (i.e., potential emulsifiers).
Fig. 7 shows the residue obtained after freeze drying 1 l. of process water.
Freeze drying sampled process water made residue visible (Fig. 7).
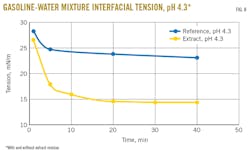
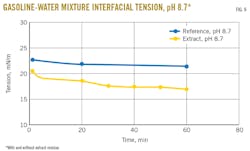
Interfacial tension between water and pygas was determined with addition of the extract residue (tested concentration: 1,000 ppm vs 100 ppm in process water) to confirm the surface-active properties of the material. Results indicated that interfacial tension is lower once the material is added to the water-gasoline, demonstrating its strong surface-active properties, most notably at lower pH (Figs. 8-9).
The dual-technique analyses (LC-MS, DIP-MS) of residue material identified specific compounds that could be responsible for emulsion stabilization: polyethylene glycol (PEG, with different end groups); fatty acids and sulfonate-type compounds; and large styrenic-fulvenic types of molecules. While the origin of such species remains under investigation, it is possible that the PEG, fatty acids, and sulfonate-type compounds are a result of recycling-process additives to the QWT or related to feedstock oxygenates, whereas the large styrenic-fulvenic compounds could be formed and inherent to the cracked-gas stream. Overall, the combination of these three types of functional groups fit with an electrosteric stabilization mechanism.
Path ahead
Based upon findings of our study, zeta and streaming potential allows quantification of the electrostatic component of the OW emulsion stabilization. Operators can use these data to help find the appropriate root cause of emulsion and fine tune a proper treatment plan for naphtha-cracking operations, such as decreasing or increasing emulsion-breaker concentration or finding the optimum pH range.
This type of analysis, we believe, brings a major benefit compared to the classical conductivity-turbidity-pH analyses usually performed by the industry on such a system.
The authors
Fabrice Cuoq ([email protected]) has worked at Saudi Arabian Basic Industries Corp. since 2013, currently serving as a senior scientist in the technology department. During this time, he has provided chemistry support on the process and water sides to SABIC’s operating plants in Europe and Saudi Arabia. Cuoq holds a MS (2009) in material sciences from Institut National des Sciences Appliquées de Lyon, France, and a PhD (2012) in colloidal chemistry from the University of Aix-Marseille, France.
Jérôme Vachon ([email protected]) has worked at Saudi Arabian Basic Industries Corp. since 2010, currently serving as a lead scientist in the technology department. After previously providing chemistry support to SABIC’s operating plants in Europe, he now focuses on development of polyolefin material. Vachon holds a MS (2002) in chemical engineering from CPE Lyon, France, and a PhD (2006) in organic chemistry from the University of Geneva, Switzerland.