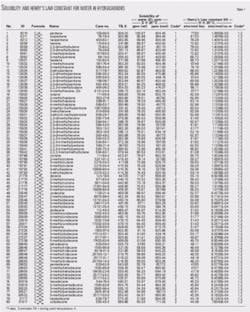
Table, correlation give water solubility, Henry’s Law constant for alkanes in crude
Water solubility and Henry’s law constant in alkanes found in crude oil have been calculated and set in an easy-to-use table.
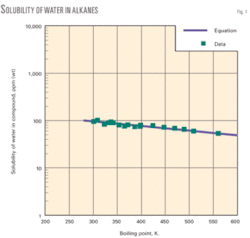
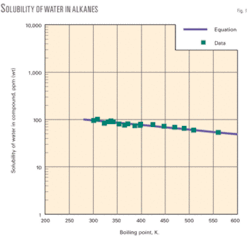
In addition, we have developed a new correlation for solubility of water in crude oil that provides reliable solubility values down to very low concentrations (ppm). The correlation is based on boiling-point temperature of the hydrocarbon. Correlation values and experimental data are in agreement.
The results are usable in engineering applications involving processing, safety, hazard, and environmental considerations.
Water solubility
The importance of the solubility of water in crude oil will increase in view of processing, safety, hazard, and environmental considerations focusing on product quality and equipment sustainability. The following brief discussion illustrates that importance.
Any processing that lowers temperatures to near the freezing point of water may result in formation of solids (freezing of water or hydrate formation). Such formation will affect both fluid flows in piping and operational characteristics of equipment. For catalytic reactions, any water in the hydrocarbon may poison the catalyst that promotes the hydrocarbon reaction.
For reactions in general, any water in the reaction species may result in formation of undesirable by-products issuing from the hydrocarbon reaction. The presence of water in the product may degrade quality and, if sufficient water is in the product, it may prove to be unusable by the customer.
This brief discussion indicates that solubility of water in hydrocarbons contained in crude oil is important in engineering applications involving processing, safety, hazard, and environmental considerations.
Correlation
Earlier works correlated the solubility of hydrocarbons and other chemical types in water as a function of the boiling point of the compound.9 11 In this work, we determined that the boiling point method was also applicable for correlation of solubility of water in alkanes:
log10(S) = A + B*TB (1)
where: S = solubility of water in compound at 25° C., ppm (wt)
TB = boiling point temperature of compound, K.
- A = 2.2740
B = –9.70 E-04
The correlation applies to a range for boiling-point temperatures of about 280 K. to 590 K.
The coefficients (A and B) for the correlation were determined from regression of the available data. In preparing the correlation, we conducted a literature search to identify data source publications.1-11 The compilations by Polak and Lu,4 Schatzberg,5 Sorensen and Artl,6 and Yaws10 were utilized for solubility of water and boiling point temperature.
We screened the publications and copied appropriate data. These data were then keyed into the computer to provide a data base for which experimental data are available. The data base also served as a basis to check the accuracy of the correlation.
The accompanying diagram shows the solubility of water vs. boiling-point temperature of alkanes. The graph discloses favorable agreement of correlation values and experimental data.
Solubility; Henry’s Law constant
The accompanying table gives the results for solubility of water and Henry’s law constant. In the tabulation, the results for Henry’s law constant are based on water solubility and vapor pressure at ambient conditions with appropriate thermodynamic relationships.10 The presented values are applicable for water in a wide variety of alkanes (normal and branching).
The results in an easy-to-use tabular format are especially applicable for rapid engineering use with a personal computer or hand calculator. The tabulation is arranged by carbon number (C1, C2, C3, so forth). This provides ease of use in quickly locating data using the chemical formula.
Examples
The results for solubility and Henry’s law constant are useful in engineering applications involving water in alkanes, per the following examples:
- Example 1. In hydrocarbon processing, hexane (C6H14) comes into contact with water at ambient conditions (25° C., 1 atm). The organic and aqueous phases are subsequently separated. Estimate the concentration of water at saturation in the hexane after separation.
Substitution of the coefficients and boiling point temperature into the correlation equation yields:
- log 10(S) = 2.2740 – 9.70 E-04*341.88 = 1.9423
S = 87.57 ppm (wt)
- Example 2. A hydrocarbon spill of hexane (C6H14) into a body of water at ambient conditions (25° C., 1 atm). After separation, the concentration of water in the hexane at the surface is 0.00033 mol fraction (xi = 0.00033). Estimate the concentration of water in the vapor at the surface.
From thermodynamics at low pressure, the vapor concentration is given by yi = Hi/Pt * xi
Substitution of Henry’s law constant from the table, total pressure (Pt = 1 atm), and liquid concentration into the above equation provides yi =72.82/1 * 0.00033 = 0.0240 = 2.40% (mol).
References
The authors
Carl L. Yaws ([email protected]) is professor of chemical engineering at Lamar University, Beaumont, Tex. His research interests include technology development; thermodynamic, transport, and environmental property data. He holds a BS in chemical engineering from Texas A&I University and an MS and PhD in chemical engineering from University of Houston.
Manish Rahate is working on a masters in environmental engineering at Lamar University, where he holds an engineering scholarship in the civil engineering department. His research interests are in thermodynamics, environmental engineering, and water treatment and purification. He holds a bachelors from Visvesvaraya National Institute of Technology, Nagpur, India.