Examples illustrate sour-gas processing megaproject metrics
Nick Amott
Stephen Mogose
Fluor Ltd.
Hampshire, UK
Increased recovery of hydrocarbon resources containing high amounts of H2S has spurred ongoing development of projects to gather and process large volumes of extremely sour gas. As a result of their physical size, capacity, and quality of feedstock, these megaprojects involve unique process and design considerations operators must address to ensure technical feasibility and potential profitability.
While initial megaprojects tested boundaries on cost, size, and technology requirements, they also provided opportunities for overcoming common problems associated with handling large volumes of sour gas, helping establish design and operational precedents now in place for future projects of this scope.
To develop the first of these sour gas megaprojects, Tengizchevroil LLP engaged consortia led by Fluor Ltd., Hampshire, UK, to provide a mix of front-end design (FEED) and engineering, procurement, and construction (EPC) services for its Tengiz asset development in West Kazakhstan. This experience and Fluor's global reach leveraged work for Abu Dhabi National Oil Co. (ADNOC) subsidiary Abu Dhabi Gas Development Co. (Al Hosn Gas) on the recently commissioned Shah gas development in the United Arab Emirates (OGJ Online, Apr. 26, 2016).
Using Fluor's direct experiences on the Tengiz and Shah developments, this article describes a selection of key metrics that led to successful conception, design, and execution.
Project drivers
Because of large investments required to build complex plants for gathering and processing sour gas, development of resources containing significant amounts of H2S historically has lagged. Shah field and its associated $10-billion processing plant were on hold for almost 50 years, while Tengiz remained undeveloped until the late 1980s.
Projects of this magnitude had to await improved technologies for handling sulfur as well as evolution of definitive end-user markets to justify operators' investments.
A particular project's strategy and design always will be influenced by several factors, including geography, access to markets (gas infrastructure), process-fluid production types, and location-specific product values.
Even within the same region, however, project drivers vary. In some remote Middle Eastern locations, thin regional demand and transportation constraints limit rates of return on finished products, offering little incentive for operator investment in large-scale sour projects. At other locations, with acute shortfalls in gas availability but large sour reserves, the production, sweetening, and export of gas can serve as megaproject drivers, with produced liquids becoming an additional revenue stream to help project economics.
Current projects in the UAE and Commonwealth of Independent States (CIS), particularly Central Asia, require processing well fluids containing up to 10 mole % CO2 and 23 mole % H2S, with future projects potentially to see higher H2S levels. Alongside handling production of oil and condensate, these projects are configured to treat and process sour gas flows up to 1 bcfd and recover NGLs and LPGs. These sour gas megaprojects also include the world's largest sulfur plants, capable of treating up to 10,000 tonnes/day of elemental sulfur.
Primarily an oil development, Tengiz always has had difficulty making production and export of gas economic due to costs associated with pipeline transportation of finished product to market.
Major Tengiz expansions during the past 10 years have therefore used conventional amine sweetening units and sulfur recovery units (SRU) to extract and convert the gas stream's H2S into sulfur.
The Tengiz Second Generation Project (SGP) used super high-pressure compressors to pioneer reinjection of 275 MMcfd sour gas (25 mol % H2S) at 620 barg as a reservoir pressure-maintenance and miscible-flood agent. Future Tengiz developments under design, and for which the procurement phase was recently approved (OGJ Online, July 5, 2016), will rely solely on sour-gas injection as their gas-disposal method.
Fig. 1 shows the SRU and tail-gas treating unit (TGTU) installed to improve plant economics at Tengiz SGP.
In the Middle East, where produced or associated gas has a far higher market value, megaproject drivers focus on sweetening gas for export as well as dealing with the low-pressure stream of acid gas resulting from sweetening.
While conversion to elemental sulfur is the most widely applied choice for handling this byproduct gas, acid gas injection (requiring compression from very low to significantly higher pressures required for injection into a disposal or producing formation) is another possible option. Its complexity, however, has limited acid gas injection primarily to Canada.
Sulfur forms
The primary focus in sour reservoir development is H2S due to its toxicity and relatively high concentration in many sour fields, which can require major additional investments for gas treating, SRU, or sour gas injection operations.
In some regions reservoirs also are prone to organic sulfur species occurring predominantly as mercaptans (RSH) of varying chain lengths but also as carbonyl sulfide (COS).
RSH compounds' toxicity and odor require the light mercaptans (methyl, ethyl, and sometimes propyl) and COS to be limited by specification. Amine sweetening, however, does not necessarily extract organic sulfur as effectively as it extracts H2S.
As the concentration of H2S in reservoir fluid increases, there also is a tendency (though not a direct correlation) for levels of organic sulfur to increase. Geography and geological history play a strong role in this situation, but since highly sour fields tend to be deep, high-pressure reservoirs (Tengiz is 4,500 m deep with 550 bar shut-in pressure), this can be a major concern for project developers. As the concentration of organic sulfur species increases, the objective shifts from needing to recognize and report sulfur levels to requiring removal of organic sulfur to meet specifications.
As the level of sulfur in produced fluid rises, elemental sulfur can also emerge. This sulfur leads to specification problems, but more fundamentally, it can lead to production issues resulting from blockage as the sulfur condenses and then deposits as a solid. Typical coproduction of water also creates a high risk of aggressive wet-sulfur corrosion. The base propensity to deposit elemental sulfur does not occur in some fields in spite of high H2S levels because coproduced liquid hydrocarbons (such as oil or heavier condensates with naphtha-like constituents) can act as a solvent for sulfur and prevent it from depositing by keeping it in solution in an elemental form. This characteristic is used by new technologies designed to control the risk of elemental sulfur deposition.
Table 1 shows the range of sour gas compositions and relevant contaminants.
Sour-gas treatment
While several technologies are available to treat sour-gas streams, the workhorse for many years has been amine solvents. Use of these solvents generally entails absorption of acid gas in a solvent solution and subsequent solvent regeneration by heating. This heating, which uses large amounts of steam, requires utilities not typically found at sites processing sweet gas or light oil.
Application of other technologies primarily depends on contaminant concentrations. Other solvents, such as methanol or a generic group known as physical solvents (vs. chemical solvents like amines) are sometimes used
Other niche applications use molecular sieves to adsorb the contaminant, requiring regeneration to release sulfur compounds and then further treatment of regeneration gas.
Solid absorbents, or scavengers, also are applicable for levels of contamination low enough to allow disposal of the scavenger. Liquid scavengers can be applied as well.
Diluted caustic (NaOH) reacts with the sulfur species, but also produces byproducts that must to be dealt with later.
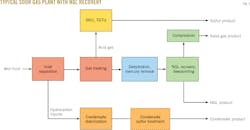
Fig. 2 shows the configuration of a typical sour-gas processing plant equipped with NGL-recovery.
Fields with highly sour reservoirs typically use amines, physical solvents, or a combination of the two for sour-gas treatment.
Amines remove H2S from the gas well, with some modified amines better suited for CO2 removal. Amines, however, are not good at removing organic sulfur compounds, particularly mercaptans. Varying percentages of removal can occur, but high-removal rates are beyond the capability of amines in tight-specification environments. Simulation can predict mercaptan removal by an amine, but not accurately.
The best prediction method uses existing plant operational data, benchmarking the simulation where possible by developing a set of binary interaction parameters or adjusting simulation parameters to emulate the operating plant.
Physical solvents have an inherently superior ability to absorb mercaptans and often are more appropriate for achieving a specification limit.
While using both solvent systems in series can be effective, it raises costs. Full removal of COS is also best achieved by absorption in an amine, particularly diglycolamine (DGA). Another effective approach uses a hydrolysis reaction to convert COS to H2S in the presence of aqueous amine. This reaction, however, is slower and best promoted by additives or catalyst.
Amines are used as aqueous solutions of varying concentrations, while physical solvents are pure and nonaqueous. The hydrolysis reaction, therefore, cannot occur with a physical solvent alone.
Amines are effective in H2S removal and can assist in removing COS, but a physical solvent is required for deep removal of mercaptans unless a subsequent unit dedicated to their removal is added in series. This can be either a molecular sieve with regeneration gas cleanup or a caustic treatment enhanced by a catalyst, most commonly the proprietary Merox treating process.
Another option is to blend the two solvent types in one circulating mixture to create a hybrid solvent, often a combination of proprietary enhanced aqueous amine solutions with additives and physical solvents offered by various technology licensors. Hybrid solvents, however, present additional problems by absorbing CO2 in the gas. This may be desirable, however, particularly for cases in which a heating-value specification for the gas can only be met if CO2 is partially removed.
The physical solvent component of the hybrid mixture will remove essentially all CO2, creating side effects in the downstream acid gas processing unit (typically SRU) and reducing the volume of treated export gas. If this gas is sold on a volume rather than calorific-value basis, the CO2 removal may affect project economics.
Physical solvents can coadsorb heavier hydrocarbons in sour gas. Since most sour gases are produced in the presence of condensates or oil, the sour gas will be rich in heavier hydrocarbons that will end up in the acid gas. This can lead to SRU-catalyst fouling and poor-quality sulfur.
Physical solvents can also absorb aromatics such as benzene, toluene, and xylenes (BTX) naturally present in well fluids, not always a desired outcome.
Table 2 shows the generalized performance of solvents when treating gas containing secondary contaminants beyond the prime basic acid gases H2S and CO2.
Highly sour-gas megaprojects require massive train throughputs for acid-gas removal units (AGRU) and especially SRUs. Tengiz SGP's single-train, 380-MMcfd amine unit, which continues to treat 16 mole % H2S content of gas with a 2,000-tonne/hr amine circulation rate, was the largest unit-design possible upon its 2008 startup.
The massive amine units at the Shah gas development, which treat 1 bcfd of gas containing 23 mole % H2S and 10% CO2 in four trains at a similar amine circulation rate to Tengiz, also were designed to maximum possible capacities. Shah's amine units yield 500 MMcfd of treated gas (after acid-gas removal). SRUs at the project produce 9,200 tonne/day of sulfur.
SRU design implications
Designing SRUs to handle production from large sour-gas fields requires consideration of four major factors:
• Increasingly strict environmental performance parameters.
• H2S-to-CO2 ratio.
• Heavy hydrocarbons in the acid gas feed.
• Overall capacities of the megaproject and its benchmark units.
The SRU combusts the H2S-rich acid gas in a reaction furnace (RF) before progressively converting it to elemental sulfur in the multistage Claus reactors and sulfur condensers. Treatment in a TGTU and a tail-gas incinerator follow.
Environmental performance parameters for SRUs, however, have become more stringent globally, moving from 93% sulfur recovery requirements for simpler Claus SRUs to current requirements of 99.9% sulfur recovery. This demands increasingly sophisticated tail-gas treating operations.
Heat energy generated from the RF, sulfur condensers, and incinerators is used in the heat-consuming TGTU in the form of steam that, like the AGRU, leads to a key additional utility system for water treatment and steam generation.
SRU combustion in the reaction furnace primarily relies on the heating value of acid gas to achieve good combustion. If BTX, for example, also is present in the acid gas, then higher temperatures are required to ensure BTX destruction. A similar constraint applies for ammonia destruction from sour water strippers, but this is more prevalent in refinery units.
While H2S has an inherent (albeit relatively low) heating value, CO2 has none. This means that an acid gas stream with too high a concentration of CO2 will not achieve satisfactory combustion, particularly in cases where ratios of CO2 exceed roughly 50 mole %.
Ways around this constraint exist, depending on the degree to which the acid gas is fuel-poor. Simple measures include preheating acid gas and combustion air, or adding supplemental fuel gas to increase the heating value. More sophisticated measures include bypassing certain feeds around the furnace, using oxygen enrichment to reduce the inerts content of combustion air, or using acid gas enrichment (AGE), which involves removing some amount of CO2 from the acid gas.
The simpler solutions quickly present limitations, as either capacity or the degree of shortcoming in heating value increases. Using fuel gas to increase heating value partly negates the gas plant's main objective of producing sales gas.
Oxygen enrichment typically serves to increase overall SRU capacity or debottleneck existing plants by reducing the volume of inerts present in air passing through the process that otherwise would increase both equipment size and pressure drop. Oxygen enrichment is a costly approach for smaller capacity step changes but can yield significant dividends when used for larger capacity increases.
Introducing heavy hydrocarbons present in acid gas to the SRU impacts both the unit's catalyst performance and the quality of its sulfur output. While a typical Claus sulfur plant yields a premium-quality, bright-yellow sulfur, heavy hydrocarbons present in acid feed gas will combust and taint the yellow sulfur, creating a harder-to-sell brown product.
More critically, these hydrocarbons deposit on the Claus catalyst, causing premature deactivation. Typically, Claus catalyst loading will ensure full run-length between scheduled plant turnarounds (roughly 4 years). While the catalyst performs well at the start of a run, premature fouling accelerates catalyst degeneration to a near end-of-run state, requiring early shutdown and catalyst change.
Probably the most important consideration in SRU design for sour-gas megaprojects is handling massive amounts of sulfur using the smallest possible number of trains. The Tengiz SRU pushed the capacity envelope to 2,350 tonnes/day by using twin parallel RFs to overcome RF-size challenges and constraints on the single-burner design scale (Fig. 3). Based on benchmarks established by the Tengiz project, the Shah plant includes four parallel SRU trains, each equivalent in capacity to one of the Tengiz plants.
High temperatures
Solvents used in both the AGRU and TGTU function optimally at temperatures that usually cannot be achieved solely with air cooling at ambient temperatures much above 35° C. Since cooling water in many hot, arid climates is either rare or nonexistent, adequate cooling in summer months with a reasonable temperature approach and exchanger design can only be achieved by supplementary chilling, which requires refrigeration.
On a sour-gas megaproject, these refrigeration requirements develop into huge systems requiring massive refrigeration compression powers, air-cooled condensers, and large piping and vessel inventories. This either adds to power-generation requirements or requires adding large gas-turbine driven compressors.
Optimization of amine systems to limit chilling duty can result in substantial savings when done early in design simply by recognizing the size of the duty. This optimization may drive the need for specialist and proprietary solvents or other open-art or patented techniques or configurations.
The TGTU quench system has particular potential for optimization. While heat removal is achieved by water circulation, this also requires cooling that typically can be accomplished with air coolers.
Heavier hydrocarbons in the sour gas entering the AGRU also have knock-on effects in the unit's solvent system, including promotion of foaming, excessive flash-gas generation in the rich solvent, and, most critically, absorption into the acid gas, which will negatively impact SRU performance.
One remedy to this issue is reducing heavy hydrocarbon content upstream of the AGRU absorber. Chilling at this point in the process must consider hydrates and associated approach temperatures. This chilling will add to overall refrigeration load but can yield marked benefits in processing configuration and plant performance.
Safety
Alongside proven and reliable technologies, a primary consideration of design impact on safety is site location and plant layout.
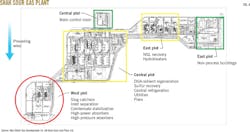
Preliminary analyses of absolute H2S concentration and partial pressure can help define potential leak scenarios as well as the scope of hazardous zones. Operators often use color codes while planning individual plant areas to graphically differentiate between zones.
Although the limits and use of coding may vary for each plant owner, specific colors are assigned to identify processing zones and designate a far wider emergency protection zone, a radius within which the public is restricted from entering.
Layout may also account for required distances between high-risk (red) and medium-risk (yellow) zones (Fig. 4), typically in relation to simultaneous operation distances or separation required between an operating part of the high-risk plant and a unit or item on which work is planned.
This approach opens up the plant layout and increases plot-space requirements, separating areas of the plant that do not contain toxic or flammable fluids at a sufficient distance from risk zones to establish areas for unrestricted worker access during normal plant operation (Fig. 5).
Design also should include identification of other safety considerations, including distances relating to possible vapor-leak dispersion, proximity to safety equipment, stairway systems (Fig. 6), safe refuges, and emergency exit pathways.
Bibliography
Aimard, A., Lallemand, F., and Rocher, A., "Sour Gas Production: Moving From Conventional to Advanced Environmentally Friendly Schemes," Society of Petroleum Engineers Annual International Oil & Gas Conference and Exhibition, Beijing, Dec. 5-7, 2006.
Al Maskari, S.M., Hughes, M., Rácz, A., Slavens, A., and Street, R., " Update on the Start-up of the World-Class Sulfur Recovery Facilities at GASCO's Habshan 5 Integrated Gas Development (IGD)," Sour Oil & Gas Advanced Technology, Abu Dhabi, Mar. 23-27, 2014.
Aljazzar, G., DeBest, M., and Graham, C., "New Elemental Sulfur Solvent Regeneration Process for Large Scale Sour Gas Plants," Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, Nov. 10-13, 2013.
Allan, K., and Street, R., " Supersize Me - Worldscale SRU/TGTU goes Operational in Kazakhstan," Gas Processors Association Europe Annual Conference, Sitges, Spain, May 13-15, 2009.
Almuhairi, A.H., Kassamali, N., Nielsen, D., and Schulte, D., "The Shah Gas Development (SGD) Project - A New Benchmark," Annual Convention of the Gas Processors Association, Austin, Mar. 21-24, 2010.
Almuhairi, A.H., Mangas, R., Melton, H., Ross, I., Sahoo, T., and Schulte, D., "The Shah Gas Development Project - A Systematic HSE Approach to Sour Gas Processing," Sour Oil & Gas Advanced Technology, Abu Dhabi, Mar. 29-30, 2011.
Amott, N., and Mogose, S., "Typical Process Challenges and Configurations for Sour Gas Mega Projects," Society of Petroleum Engineers Kuwait Oil & Gas Show and Conference, Mishref, Kuwait, Oct. 11-14, 2015.
Amott, N., "Shah Field Development Overview," Institution of Chemical Engineers Sour Gas Seminar, London, Oct. 30, 2013.
Amott, N., "Shah Field HSE Challenges," Institution of Chemical Engineers Sour Gas Seminar, London, Oct. 30, 2013.
Amott, N., Block, M., and Lanterman, J., "Sour Gas Injection Design Extends the Envelope in Kazakhstan," Gas Processors Association Europe Annual Conference, Warsaw, Sept. 21-23, 2005.
Amott, N., Connell, D., Cullum, I., and Ormiston, B., "Challenges of a Complex Gas Plant Revamp in Kazakhstan," Annual Convention of the Gas Processors Association, Atlanta, Mar. 12-15, 2000.
Armstrong, L., and Roberts, P., "Challenges for Processing Oil and Gas from Kazakhstan," Gas Processors Association Europe Annual Conference, Warsaw, Sept. 21-23, 2005.
Bignold, M., Cheng, N., and Hanlon, G., "Shell Canada's Caroline Gas Plant Reaches Smooth Operation," 47th Laurance Reid Gas Conditioning Conference, Norman, Okla., Mar. 2-5, 1997.
Carroll, J., and Foster, J., "New Challenges & Solutions in Designing Large Sour Gas Projects," Gas Arabia Summit, Abu Dhabi, Feb. 18-20, 2008.
Chow, T., Elkins, M., Madkour, S., and Wong, V., "10,000 MTD Sulfur Recovery and Tail Gas Treating Complex for the GASCO Shah Gas Development Project," Sour Oil & Gas Advanced Technology, Abu Dhabi, Mar. 31-Apr. 1, 2009.
Chow, T., Kister, H., Nielsen, R., and Sanchez, A.E., "Low Energy Direct Contact Condenser Designs for Claus Tail Gas Treating Units in Desert Environments," Sour Oil & Gas Advanced Technology, Abu Dhabi, Mar. 29-30, 2011.
Kohl, A., and Nielsen, R., Gas Purification, 5th edition, Houston: Gulf Publishing Co., 1997.
Nicholson, S., "Gas Treating and Processing Technologies," Institution of Chemical Engineers Sour Gas Seminar, London, Oct. 30, 2013.
Based on a presentation to Gas Processors Association Europe Spring Conference, Paris, Apr. 20-22, 2016.
The authors
Nick Amott ([email protected]) is a process director and senior fellow specializing in oil and gas production at Fluor Ltd. in the UK. He has been with the company for almost 35 years, holding various process engineering positions. Amott holds a BSc (1978) in chemical engineering from the University of Surrey, where he currently serves as a Royal Academy of Engineering visiting professor. He is a chartered engineer and fellow of the Institution of Chemical Engineers, a director of Gas Processors Association Europe, and a member of the Society of Petroleum Engineers.
Stephen Mogose ([email protected]) has spent his entire career with Fluor Ltd. in the UK and currently serves as executive director and lead of upstream technology for Europe, Africa, and the Middle East. He holds a BEng (1978) in chemical engineering from University College, London. Mogose is a chartered engineer with the Engineering Council in the UK and a fellow of the Institution of Chemical Engineers.