P. 3 ~ Continued - Mixing MDEA, TEA shows benefit for gas-sweetening operations
View Article as Single page
Process simulation
Different alkanolamine mixtures were simulated with the TSWEET kinetic model incorporated in the process simulator ProMax 3.0 software.18 Table 4 presents design data used for simulations for the Habshan gas unit OGD II. They were provided by GASCO.
The additive concentration (MEA, DEA, DIPA, or TEA) was varied from 0 to 20 wt % with the solvent total amine concentration fixed at 45 wt % for all the studied cases. (MDEA concentrations vary between 45 and 25 wt %.) For the first simulations, convergence of the regenerator was obtained through specification of a condenser temperature of 57o C. and a lean amine loading of 0.005 total moles of acid gas/moles of amine. Then, a final case study was carried out to investigate the effect of varying the lean amine loading on the reduction of the reboiler duty.
Results obtained for the mixture MDEA-TEA were compared in terms of performance with the other currently used alkanolamine mixtures such as MDEA-MEA, MDEA-DEA, and MDEA-DIP, and also against results obtained for the use of MDEA only. (GASCO Habshan unit OGD II uses only MDEA solvent at 45 wt %.)
Comparison between different mixtures was made based on regenerator reboiler duty, H2S and CO2 concentration in sweet gas, and rich amines' loading capacities. Also, the effects of changing the trim-cooler temperature on the absorption of acid gas by MDEA-TEA mixture were studied in order to achieve the initial H2S specification produced by the single MDEA solvent 45 wt %. Finally, the economic impact of the use of MDEA-TEA mixture (40 wt % MDEA + 5 wt % TEA) on the unit's operating costs was discussed.
Results, discussion
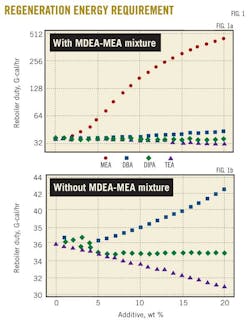
Fig. 1 shows the regenerator reboiler duty necessary to strip different rich-amine solutions from acid gases as function of the additive percentage in the 45 wt %. MDEA-X. X stands for MEA, DEA, DIPA, and TEA solutions with a percentage varying from 0 to 20 wt %.
Fig. 1a shows the dramatic increase of the stripper's energy requirement with the increase in the MEA contribution to the 45 wt % MDEA-MEA amine mixture. Results of Fig. 1a are also depicted in Fig. 1b but without those for MDEA-MEA mixture. The reason is to show the difference in energy requirements for MDEA-DEA, MDEA-DIPA, and MDEA-TEA mixtures.
As far as the addition of DIPA is concerned, a small increase and decrease in the regenerator duty occurs within the 5% addition of DIPA. Beyond this percentage of added DIPA, the stripper energy requirement becomes insensitive to the DIPA quantity used to replace MDEA in the 45 wt % MDEA-DIPA mixture. For the MDEA-DEA mixture, an increase of 16% in the duty appears when 20% of the MDEA is replaced by DEA in the 45 wt % MDEA-DEA mixture.
Fig. 1b shows that addition of TEA has resulted in a constant decrease of the regenerator duty to 14% when 20% of the MDEA is replaced by TEA in the 45 wt % MDEA-TEA solution. This can be explained based on the data provided in Table 2, which shows that TEA had the lowest heat of reaction with H2S and CO2 compared with other solvents.
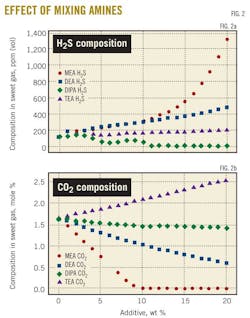
Fig. 2 depicts the effect of mixing of different amines with MDEA on the absorption of the acid gases.
Fig. 2a shows that TEA addition to MDEA causes an increase in H2S concentration in the sweet gas. DEA-MDEA and DIPA-MDEA mixtures, however, score higher H2S concentrations.
In terms of CO2 absorption, Fig. 2b shows that MDEA-TEA mixture is the worst among all the mixtures in terms of bulk removal of CO2. It is a matter of fact that all alkaline solutions are thermodynamically selective to CO2 and kinetically selective to H2S.
Table 1 shows that primary amines have the highest rate of reaction constants followed by secondary then by tertiary amines. When H2S dissolves in amine, it immediately converts to sulfide and bisulfide ions via an instantaneous and reversible protonation reaction with hydrogen ions.
Displaying 3/6
View Article as Single page